Compositions
a technology of colon cleansing and compositions, applied in the direction of drug compositions, inorganic non-active ingredients, dispersed delivery, etc., can solve the problems of poor patient compliance, copious diarrhea and colon cleansing, and failure to consume the full volume of solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sodium Ascorbate / Ascorbic Acid Solutions
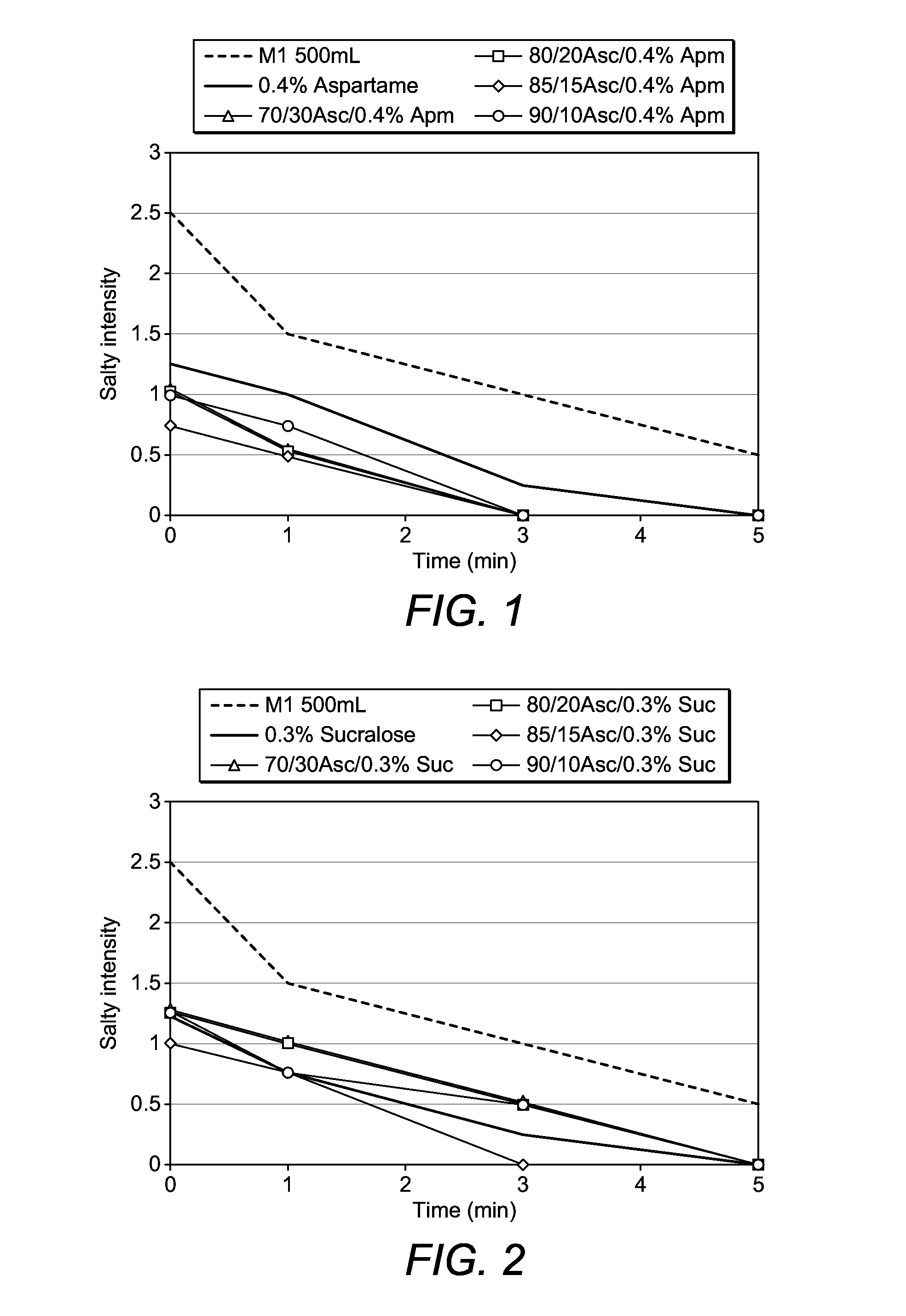
[1044]In an initial set of solutions containing PEG3350 (40 g), sodium chloride (2.8 g), potassium chloride (1.3 g) and sodium ascorbate (56.6 g), it was found that the sweeteners sucralose and aspartame were most effective in reducing the perceived saltiness of the solution. Acesulfame-K and sodium saccharin were less effective.
[1045]The solutions in Tables 1 and 2 were prepared and taste tested. The results of the taste testing are shown in FIGS. 1 and 2.
TABLE 1Aspartame-containing solutionsSodiumAscorbicWaterPEG3350 / NaCl / KCl / Aspartame / Ascorbate / Acid / Molarto Vol / Sol'nggggggratiomlF1402.81.3056.600100:0 500F2402.81.32.056.600100:0 500F3402.81.32.039.6215.1070:30500F4402.81.32.045.2810.0680:20500F5402.81.32.048.117.5585:15500F6402.81.32.050.945.0390:10500
TABLE 2Sucralose-containing solutionsSodiumAscorbicWaterPEG3350 / NaCl / KCl / Sucralose / Ascorbate / Acid / Molarto Vol / Sol'nggggggratiomlG1402.81.3056.600100:0 500G2402.81.31.556.600100:0 500G3402.81.3...
example 2
Sodium Ascorbate Solutions and Taste Testing
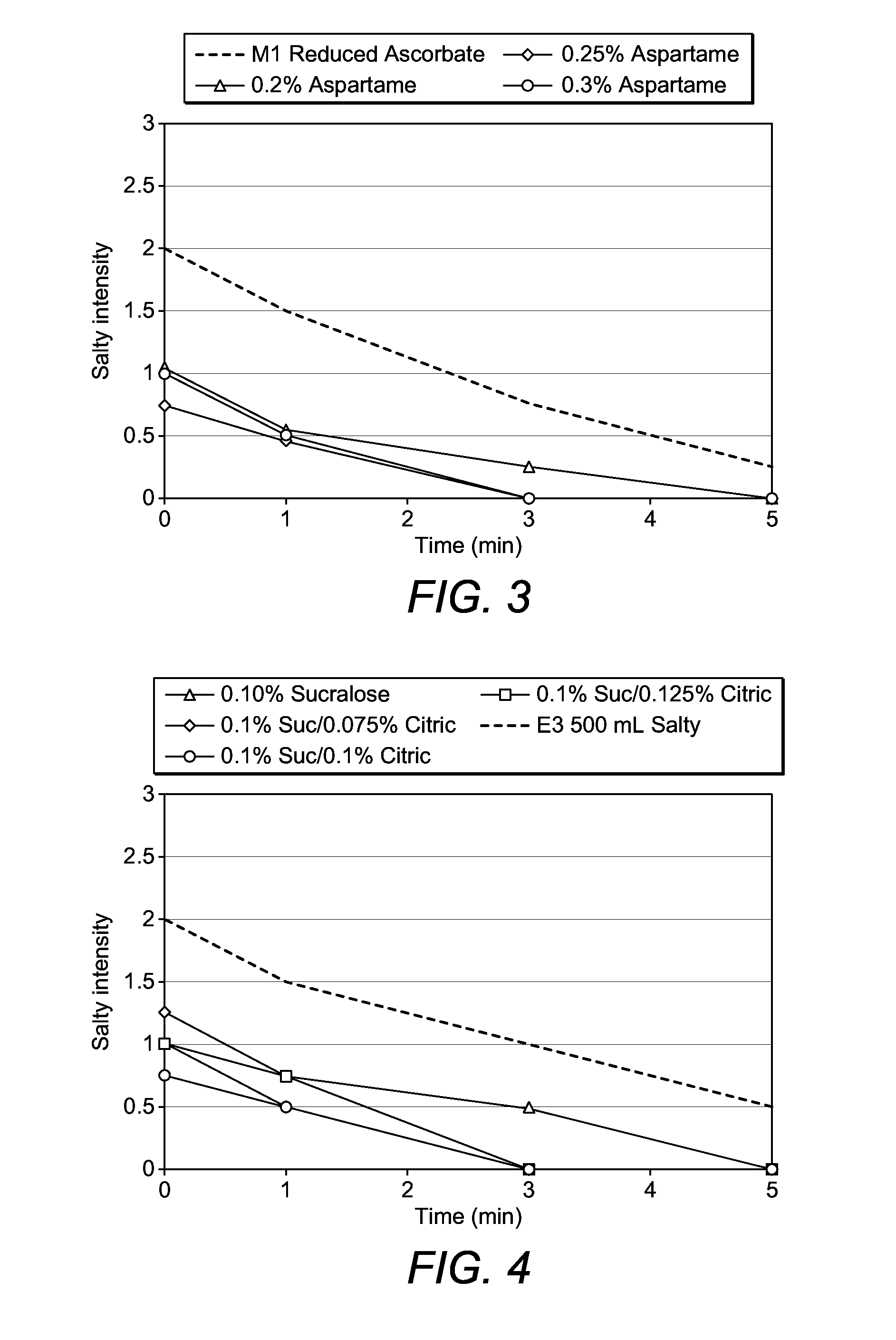
[1047]The solutions in Table 3 were prepared and taste tested. The results of the taste testing are shown in FIG. 3.
TABLE 3Sodium ascorbate solutionsSodiumWaterPEG3350 / NaCl / KCl / Aspartame / Ascorbate / to Vol / Sol'ngggggmlH1402.81.3056.6500H2402.81.3040500H3402.81.31.0040500H4402.81.31.2540500H5402.81.31.5040500
[1048]In FIG. 3, it is seen that the saltiness intensity is reduced by decreasing the amount of sodium ascorbate in the solution (compare H1 vs H2). It is further seen that the saltiness is reduced most by 1.25 g / 500 ml of aspartame.
example 3
PEG-Electrolyte Solutions and Taste Testing
[1049]In an initial set of solutions containing PEG3350 (100 g), sodium sulphate (9.0 g), sodium chloride (1.4 g), potassium chloride (0.3 g), it was found that the sweeteners sucralose (0.1%), aspartame (0.4%) or a mixture of the two (sucralose 0.07% / aspartame 0.12%) were most effective in reducing the perceived saltiness of the solution. Acesulfame-K, sodium saccharin and other mixtures were less effective.
[1050]The solutions in Table 4 were prepared and taste tested. The results of the taste testing are shown in FIG. 4.
TABLE 4PEG-electrolyte solutionsCitricWaterPEG3350 / Na2SO4 / NaCl / KCl / Sucralose / Acid / to Vol / Sol'nggggggmlI1100.09.01.40.300500I2100.09.01.40.30.500500I3100.09.01.40.30.500.375500I4100.09.01.40.30.500.50500I5100.09.01.40.30.500.625500
[1051]The results of the taste testing are shown in FIG. 4. It is seen that the solutions containing citric acid were perceived as less salty than the solutions without citric acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com