Blackened composite electroless nickel coatings
a nickel coating and composite electroless technology, applied in coatings, transportation and packaging, chemical coatings, etc., can solve the problems of multiple challenges, insoluble particulate matter in composite electroless baths, and inability to remove heavy metals, etc., to achieve the effect of cessing or reducing the plating ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0093]The following example is an illustration whereby diamond particles were codeposited in a hard metallic matrix. It should be noted that the present invention is not limited to the type of bath used herein, but in general for electroless deposits of the various metals and alloys. Moreover, the invention is not limited to the specific particles used, or method of altering the surface of the coating.
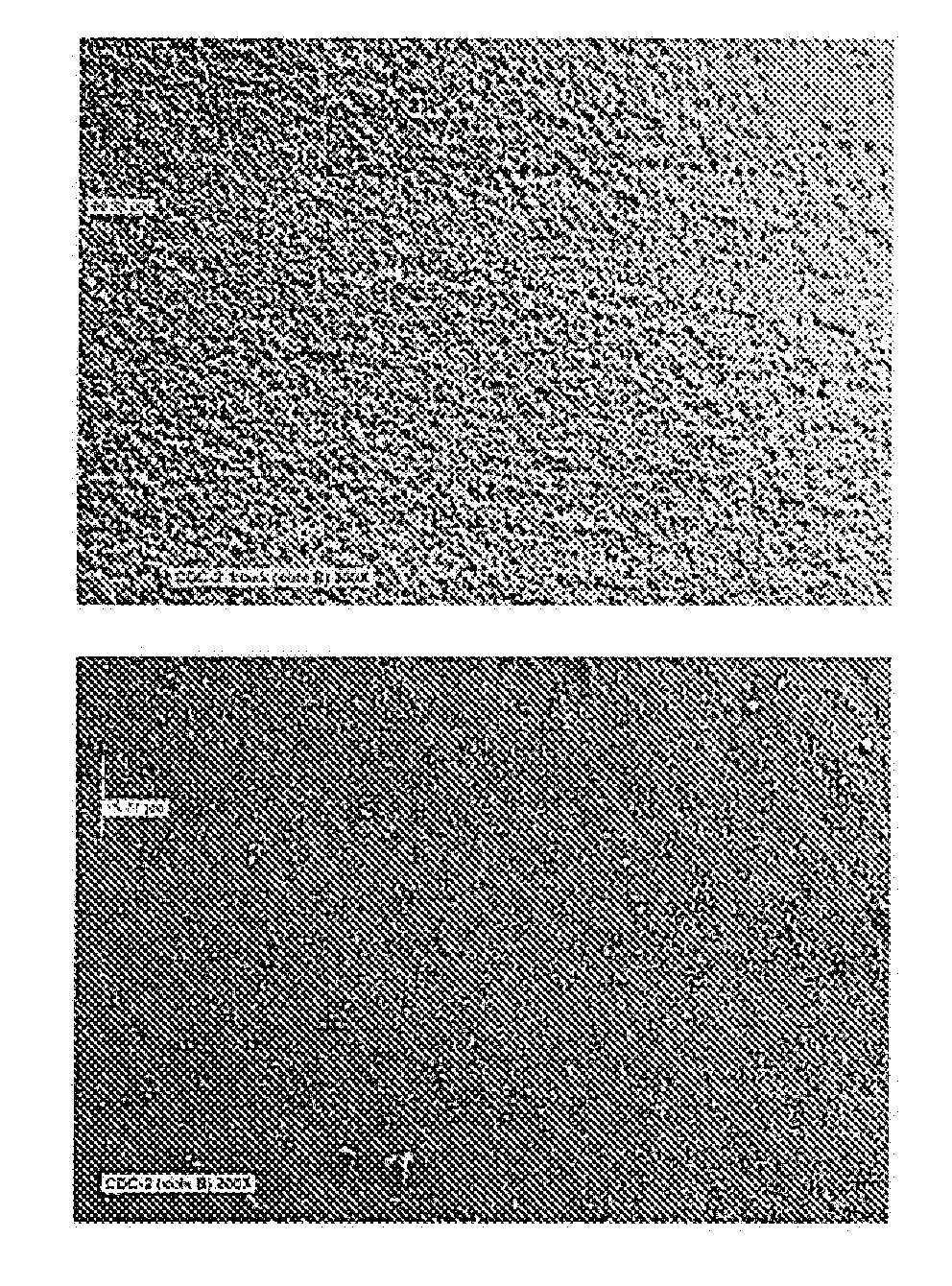
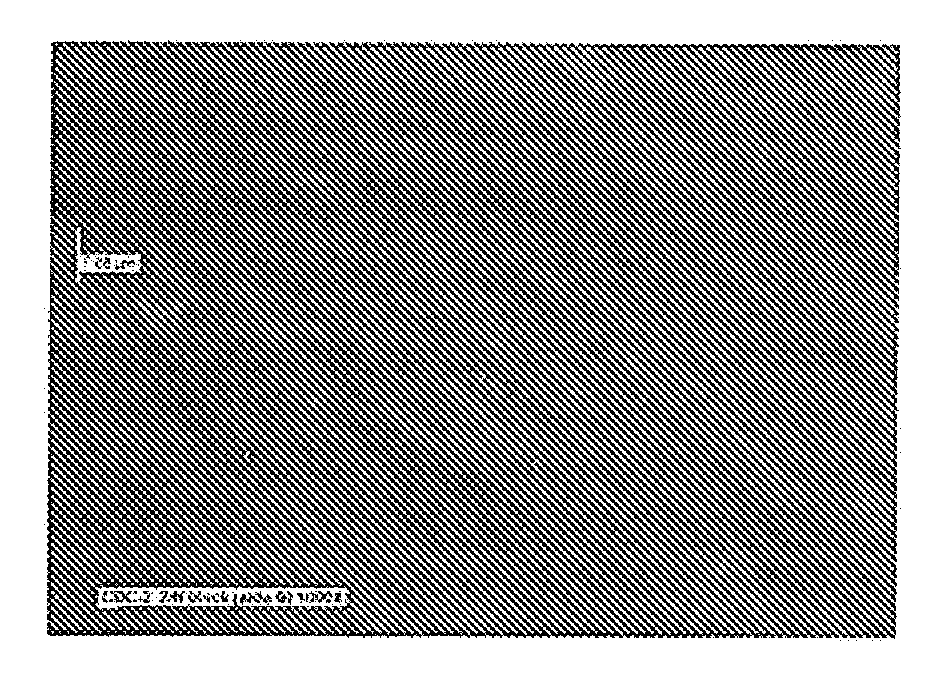
[0094]In the current example an electroless nickel plating bath, NiPlate 830 sold by Surface Technology, Inc, of Trenton, N.J., was used to provide a Ni—P type alloy with diamond particles also known as “CDC-2”. The bath was operated at a pH of 6.3 and a temperature of about 170 degrees F. Six steel panels after normal cleaning and activation were plated for 65 minutes under the above conditions. A cross sectional inspection of the coating on one of the panels revealed a significant quantity of codeposited diamond particles. Three of these coated panels were then treated by immersion i...
example 2
[0096]In the current example an electroless nickel plating bath, NiPlate 830 sold by Surface Technology, Inc., was used to provide a Ni—P type alloy with diamond particles, also known as CDC-2. The bath was operated at a pH of 6.3 and a temperature of 168 degrees F. Six steel panels after normal cleaning and activation were plated for 65 minutes under the above conditions. The coated panels were then plated with an “overcoat” of an electroless nickel coating that was approximately 2-7 microns thick and essentially free of particles. This overcoat was applied in an electroless nickel bath, NiPlate 100 sold by Surface Technology, mc, to provide a medium phosphorous Ni—P type alloy. The bath was operated at a pH of 4.8 and a temperature of 188 degrees F., and a cycle time of 12 minutes. The coated panels therefore were coated with a coating known as CDC-2 / N. A cross sectional inspection of the coating on one of the panels revealed a significant quantity of codeposited diamond particles...
example 3
[0098]The following example is an illustration whereby boron nitride particles were codeposited in a hard metallic matrix. It should be noted that the present invention is not limited to the type of bath used herein, but in general for electroless deposits of the various metals and alloys. Moreover, the invention is not limited to the specific particles used, or method of altering the surface of the coating.
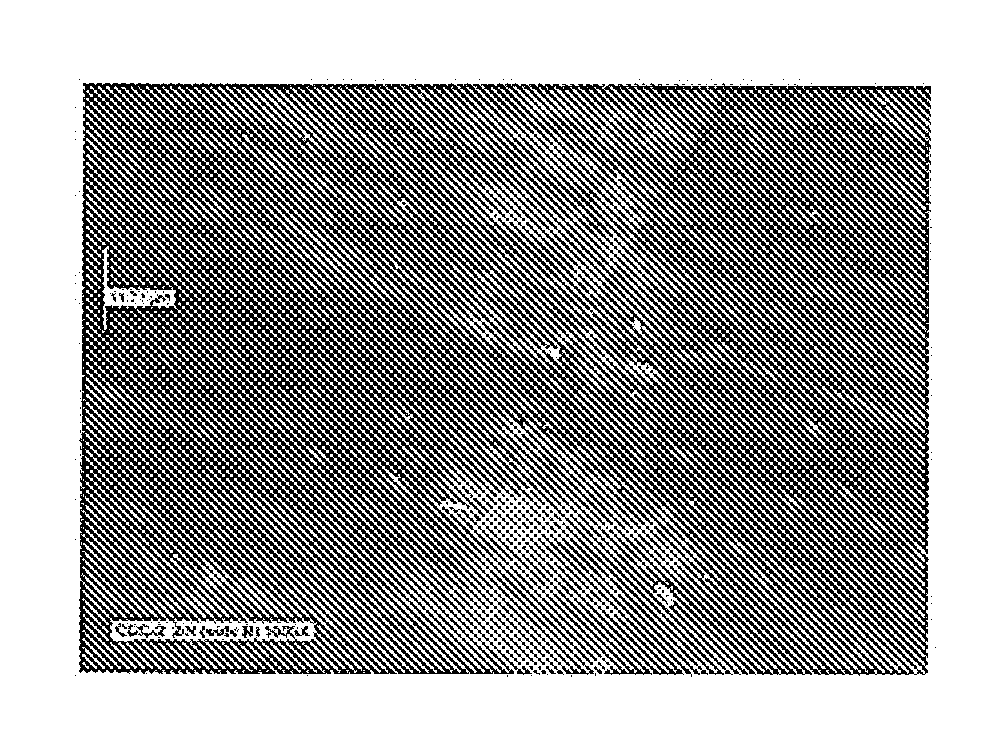
[0099]In the current example an electroless nickel plating bath, NiSlip 25 sold by Surface Technology, Inc., was used to provide a Ni—P type alloy with boron nitride particles. These particles had a mean particle size of about one micron. The bath was operated at a pH of 6.3 and a temperature of about 170 degrees F. Six steel panels after normal cleaning and activation were plated for 65 minutes under the above conditions. A cross sectional inspection of the coating on one of the panels revealed a quantity of about 10 to 15% codeposited boron nitride particles. Three of these coa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Color | aaaaa | aaaaa |
| Wear resistance | aaaaa | aaaaa |
| Energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com