Automated devices, systems, and methods for the fabrication of tissue
a tissue and automatic technology, applied in the field of health care industry, can solve the problems of low rate of new therapeutic discovery, high cost of drug discovery, and long process, and achieve the effects of reducing the urgent need for tissues, reducing the number and quality of innovative, and facilitating the application of regenerative medicin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
HASMC-HAEC Mixed Cellular Cylinders
Cell Culture
[0291]Smooth Muscle Cells:
[0292]Primary human aortic smooth muscle cells (HASMC) were maintained and expanded in low glucose Dulbecco's modified eagle medium (DMEM; Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% fetal bovine serum (FBS), 100 U / ml Penicillin, 0.1 mg / ml streptomycin, 0.25 μg / ml of amphotericin B, 0.01M of HEPES (all from Invitrogen Corp., Carlsbad, Calif.), 50 mg / L of proline, 50 mg / L of glycine, 20 mg / L of alanine, 50 mg / L of ascorbic acid, and 3 μg / L of CuSO4 (all from Sigma, St. Louis, Mo.) at 37° C. and 5% CO2. Confluent cultures of HASMCs between passage 4 and 8 were used in all studies.
[0293]Endothelial Cells:
[0294]Primary human aortic endothelial cells (HAEC) were maintained and expanded in Medium 200 supplemented with 2% FBS, 1 μg / ml of hydrocortisone, 10 ng / ml of human epidermal growth factor, 3 ng / ml of basic fibroblast growth factor, 10 μg / ml of heparin, 100 U / ml Penicillin, 0.1 mg / ml streptomycin, a...
example 2
Multi-Layered Vascular Tubes
Cell Culture
[0301]Smooth Muscle Cells:
[0302]Primary human aortic smooth muscle cells (HASMC; GIBCO) were maintained and expanded in low glucose Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U / ml Penicillin, 0.1 mg / ml streptomycin, 0.25 μg / ml of amphotericin B, 0.01M of HEPES (all from Invitrogen Corp., Carlsbad, Calif.), 50 mg / L of proline, 50 mg / L of glycine, 20 mg / L of alanine, 50 mg / L of ascorbic acid, and 3 μg / L of CuSO4 (all from Sigma, St. Louis, Mo.) at 37° C. and 5% CO2. Confluent cultures of HASMC between passage 4 and 8 were used in all studies.
[0303]Endothelial Cells:
[0304]Primary human aortic endothelial cells (HAEC) were maintained and expanded in Medium 200 supplemented with 2% FBS, 1 μg / ml of hydrocortisone, 10 ng / ml of human epidermal growth factor, 3 ng / ml of basic fibroblast growth factor, 10 μg / ml of heparin, 100 U / ml Penicillin, 0.1 mg / ml streptomycin, and 0.25 μg / ml of amphotericin B (all ...
example 3
Bioprinter
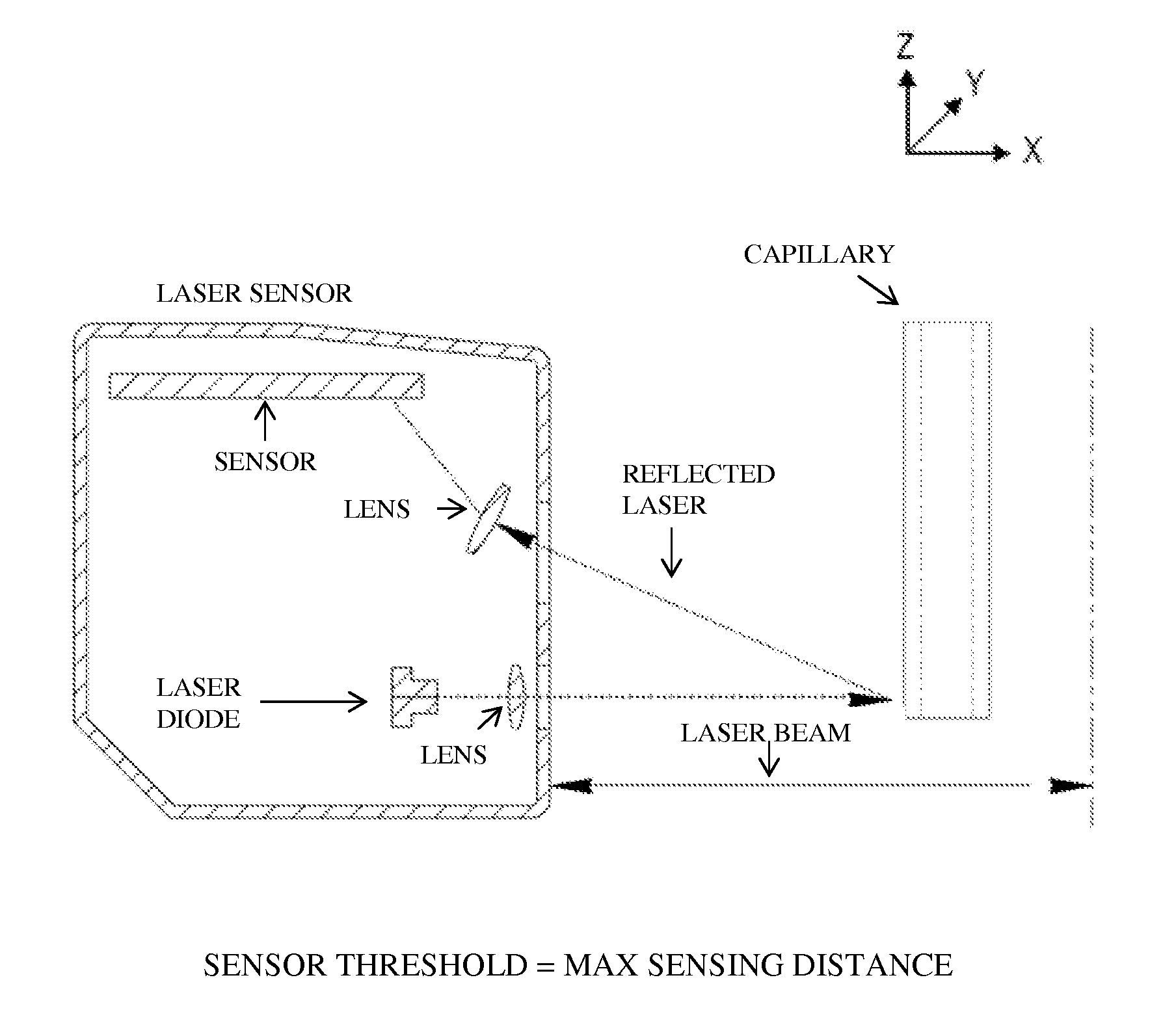
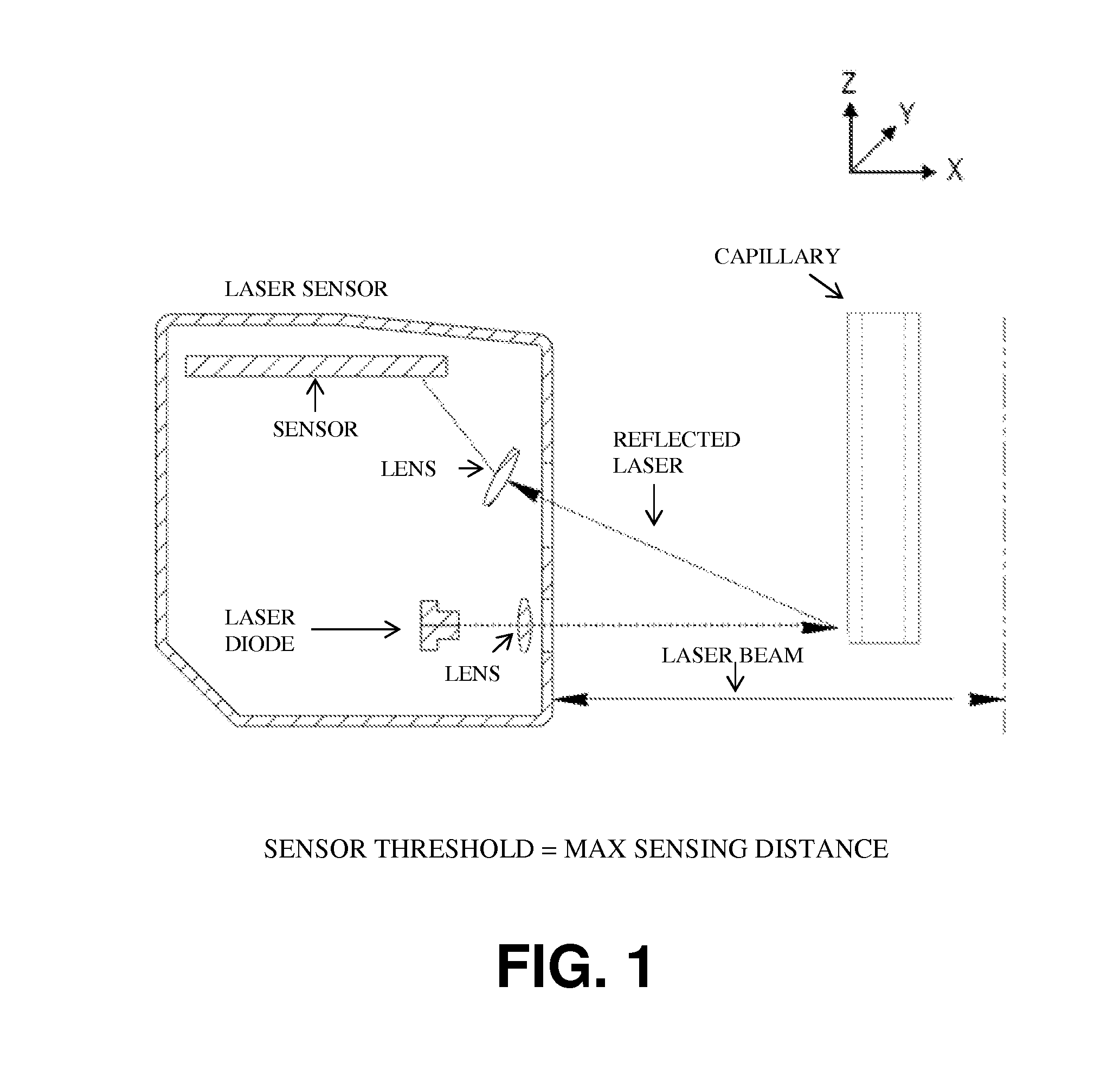
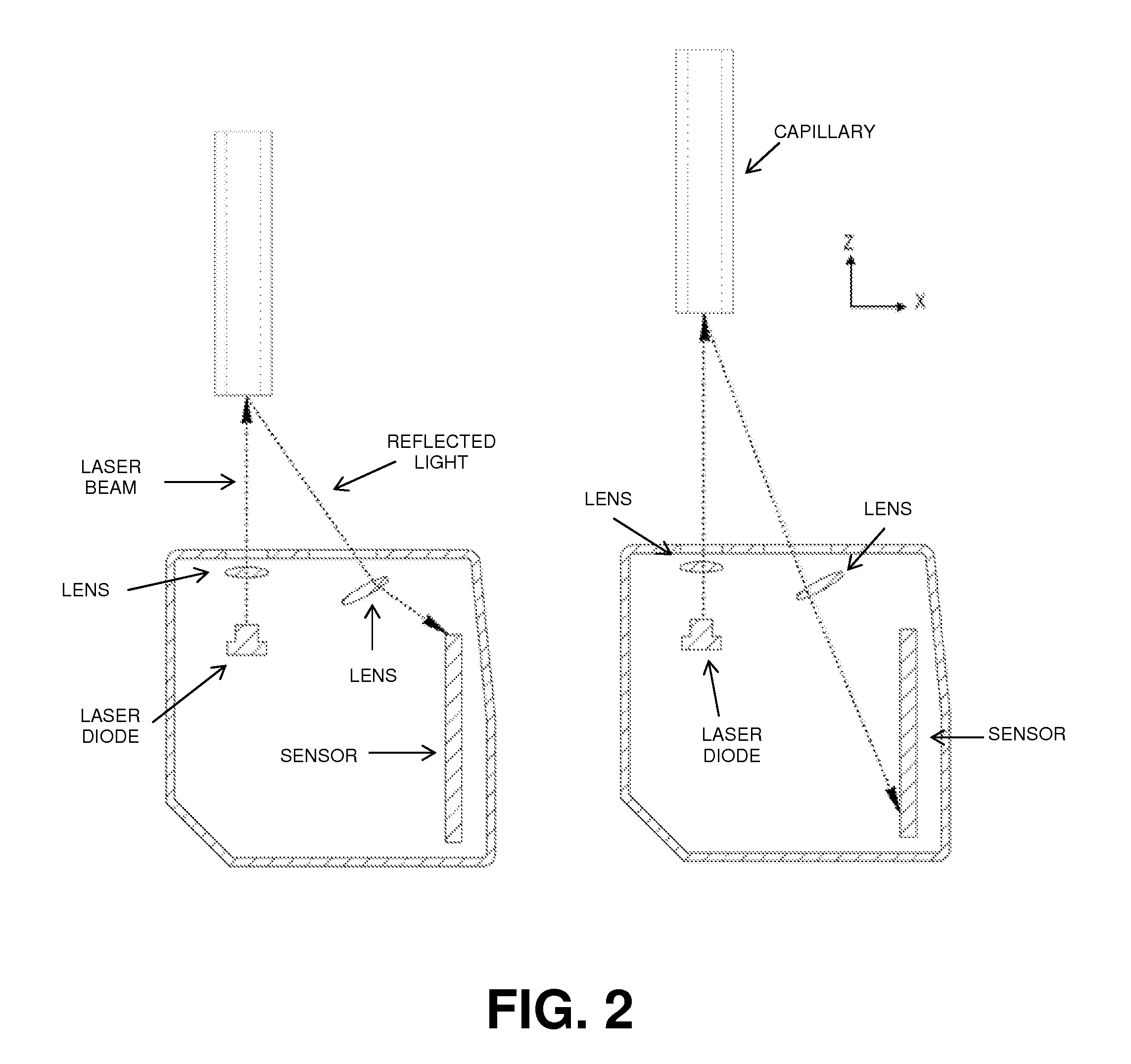
[0320]A bioprinter was assembled. The bioprinter contained a printer head having a collet chuck grip for holding a cartridge, and a piston for dispensing the contents of the cartridge. The cartridges used were glass microcapillary tubes having a length of 75-85 mm. A new capillary tube was loaded each time bio-ink or support material was required.
[0321]In order to print structures, a dispense position repeatability of ±20 μm was required for the duration of the printing process, i.e., when new capillaries were loaded into the printer head. In order to maintain repeatability of all loaded capillary tubes relative to the same point in the x-, y-, and z-directions, the bioprinter contained a laser calibration system for calibrating the position of the microcapillary tube. The laser calibration system calibrated the position of all capillary tips to a common reference location. All printing moves were made relative to this reference position.
[0322]All three axes (x-, y-, and z...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Flow rate | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com