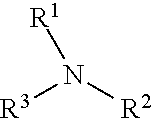
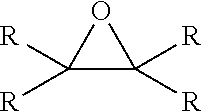
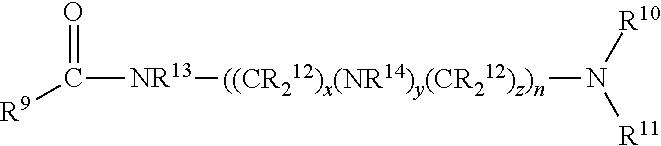Alkoxylated quaternary ammonium salts and fuels containing them
a technology of alkoxylated quaternary ammonium salt and fuel additive, which is applied in the direction of fatty acid chemical modification, machines/engines, mechanical apparatus, etc., can solve the problems of poor fuel economy, higher emissions, and components that can degrade during engine operation and form deposits, so as to improve the performance of fuel injectors, improve demulsibility, and improve the effect of deterrence performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
##ventive example 1
Inventive Example 1
[0049]A mixture of PIBSA (229 grams) and glycidol (17.4 grams) and aromatic solvent (53 grams) was heated to 45° C. for 30 minutes. Oleylamido propyl dimethylamine (86 grams) was added to the mixture slowly to keep the temperature below 52° C. Then 2-ethylhexanol (94 grams) was added to the mixture. The final mixture was reacted at 55° C. for 1 hour, then 60° C. for 2.5 hours, and 65° C. for 1 hour to give product as a viscous oil.
##ventive example 2
Inventive Example 2
[0050]A quaternary ammonium salt was prepared similarly to that of Inventive Example 1 except that a C20-C24 alkenyl succinic anhydride was used in place of PIBSA.
##ventive example 3
Inventive Example 3
[0051]A quaternary ammonium salt was prepared similarly to that of Inventive Example 1 except that dodecyldimethylamine was used in place of oleylamido propyl dimethylamine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com