Carbon dioxide reduction device and method for reducing carbon dioxide
a carbon dioxide and reduction device technology, applied in the direction of metal/metal-oxide/metal-hydroxide catalysts, physical/chemical process catalysts, instruments, etc., can solve the problem that the efficiency of reducing co2 /sub>by light energy to convert it into carbon compounds without the use of any external power source is not necessarily high enough, so as to reduce co2. , the effect of reducing co2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
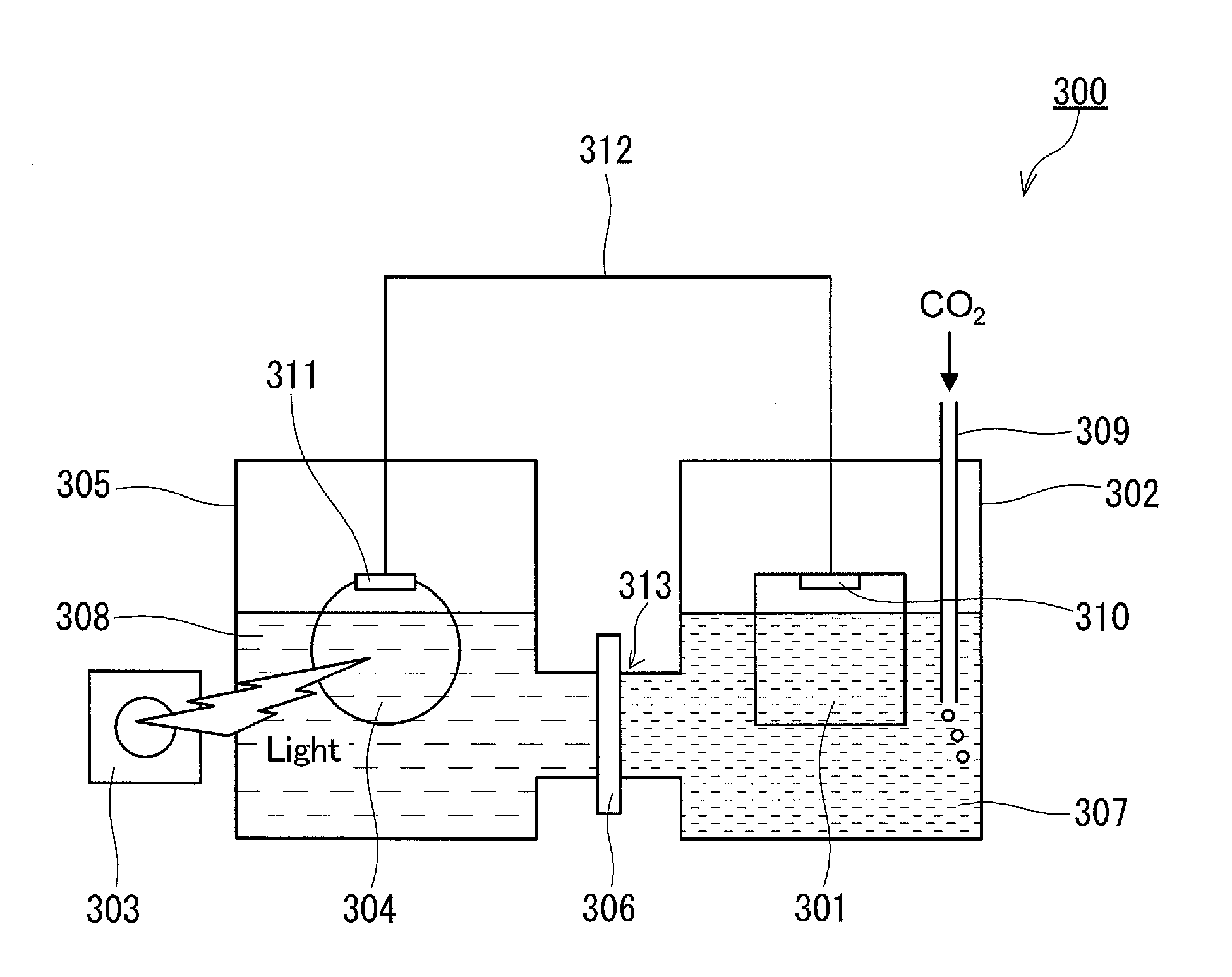
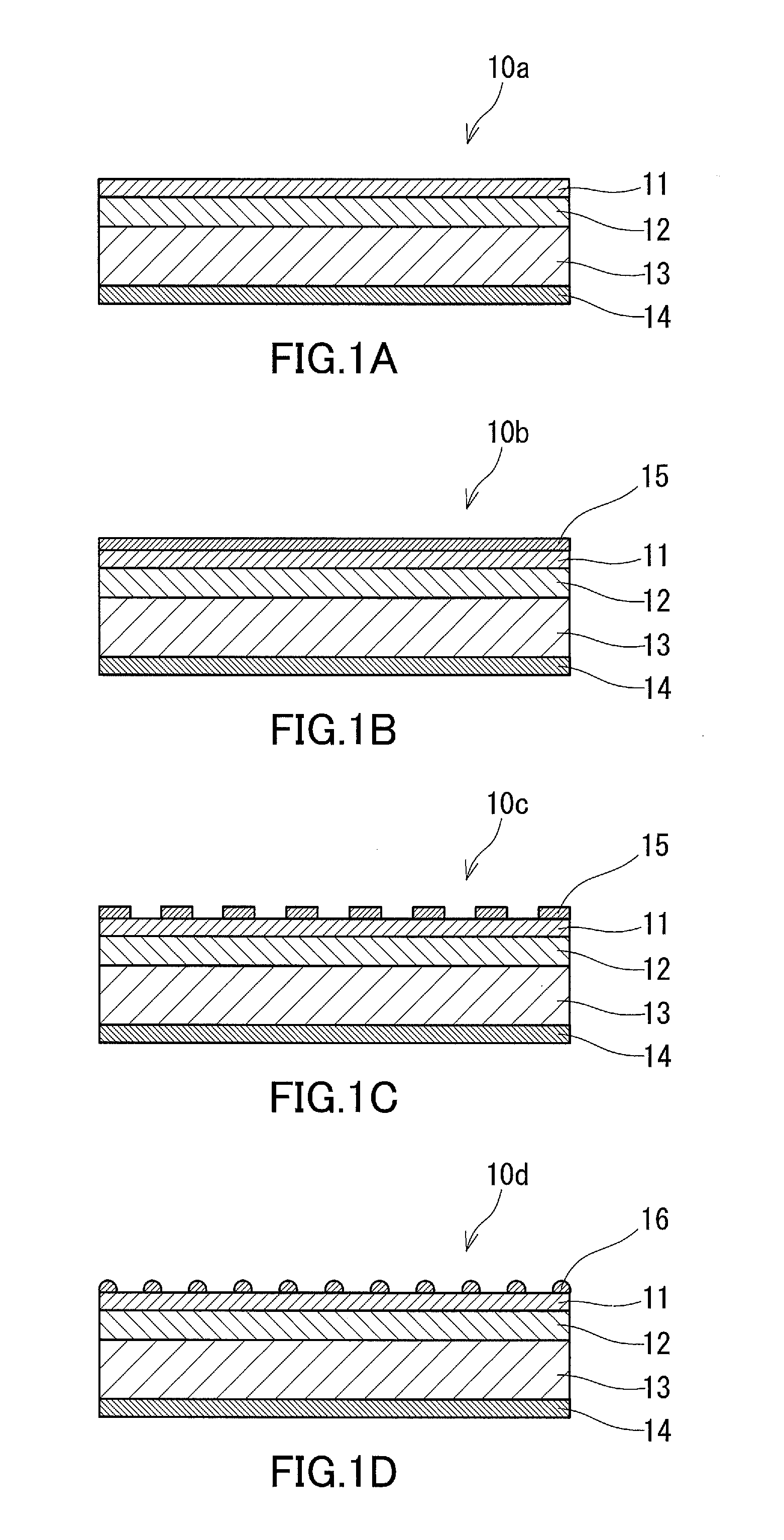
[0131]In Example 1, a multilayer body of an electrode layer, an electrically conductive substrate, a GaN layer, and a Mg-containing AlxGa1-xN layer was used as an anode electrode. On the AlxGa1-xN layer of this multilayer body (on the surface of the AlxGa1-xN layer opposite to the surface thereof facing the GaN layer), fine particles of Ni-containing metal oxide were dispersedly arranged, as shown in FIG. 1D. The electrically conductive substrate was a single-crystal GaN substrate doped with high-concentration Si (with a thickness of about 0.4 mm). The GaN layer was a Si-doped n*-type GaN layer (with a thickness of 3.0 μm and a Si doping concentration of 4.0×1018 in terms of the number of atoms per cm3). The thickness of the AlxGa1-xN layer was 100 nm, the value of x was 0.10, and the Mg doping concentration was 1.0×1017 in terms of the number of atoms per cm3. The metal oxide fine particles were fine particles of nickel oxide (with a diameter of several tens of nanometers to severa...
example 2
[0156]A CO2 reduction device was fabricated in the same manner as in Example 1, except that fine particles of nickel oxide were not arranged on the AlxGa1-xN layer of the anode electrode, and the device thus fabricated was irradiated with light in the same manner as in Example 1.
[0157]In Example 2, as in the case of Example 1, it was confirmed that upon irradiation of the AlxGa1-xN layer of the anode electrode with light, a gas was evolved from the surface of the AlxGa1-xN layer of the anode electrode and that carbon monoxide and formic acid were produced by the reduction of CO2 contained in the first electrolyte solution in the cathode chamber.
[0158]Next, the value of current flowing between the electrodes during light irradiation in Example 2 was compared with that in Example 1. The value of current in Example 2 was almost equal to that in Example 1. The amount of CO2 reduction products (carbon monoxide and formic acid) produced in a given period of irradiation time in Example 2 w...
example 3
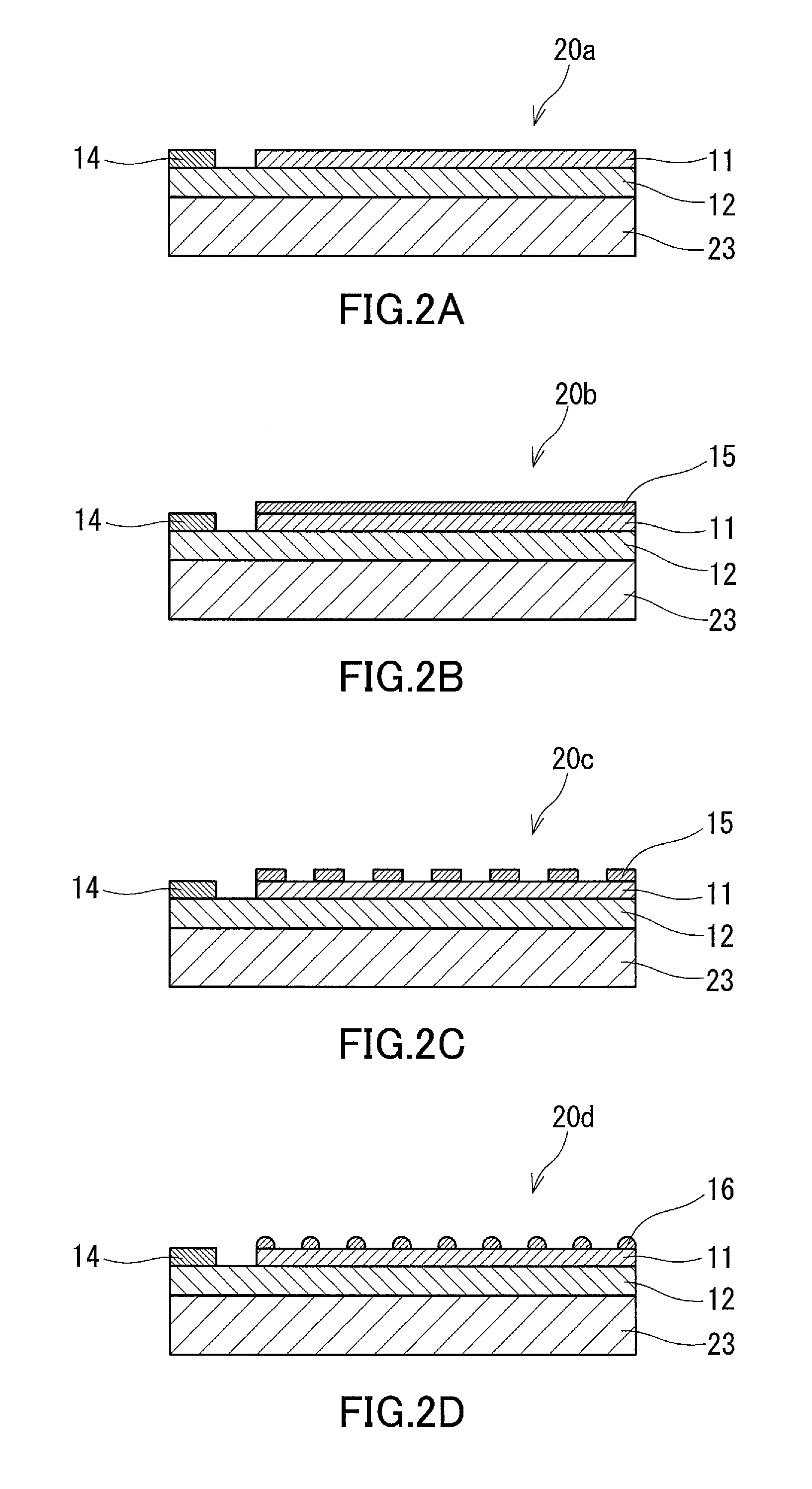
[0159]In Example 3, a CO2 reduction device was fabricated in the same manner as in Example 1, except that an insulating substrate (single-crystal sapphire substrate with a thickness of about 0.4 mm) was used instead of an electrically conductive substrate as the substrate of the anode electrode and that the electrode layer was placed on the GaN layer (see FIG. 2D) instead of on the surface of the substrate opposite to the surface thereof facing the GaN layer. The electrode layer was placed in such a manner that the Ti film was in contact with the GaN layer to increase the adhesion between the electrode layer and the GaN layer and to reduce the interfacial resistance therebetween.
[0160]The AlxGa1-xN layer of the anode electrode in the CO2 reduction device thus fabricated was irradiated with light in the same manner as in Example 1. As a result, it was confirmed that a gas was evolved from the surface of the AlxGa1-xN layer of the anode electrode and that carbon monoxide and formic ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| band gap | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com