Multimeric oligonucleotide compounds
a technology of oligonucleotide compounds and oligonucleotide reagents, which is applied in the field of oligonucleotide reagents and oligonucleotide therapeutics, can solve the problems of limiting their effectiveness as therapeutics in some cases, and achieves the effects of reducing clearance, increasing concentration, and prolonging the duration of target knockdown
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of Antisense Oligonucleotides
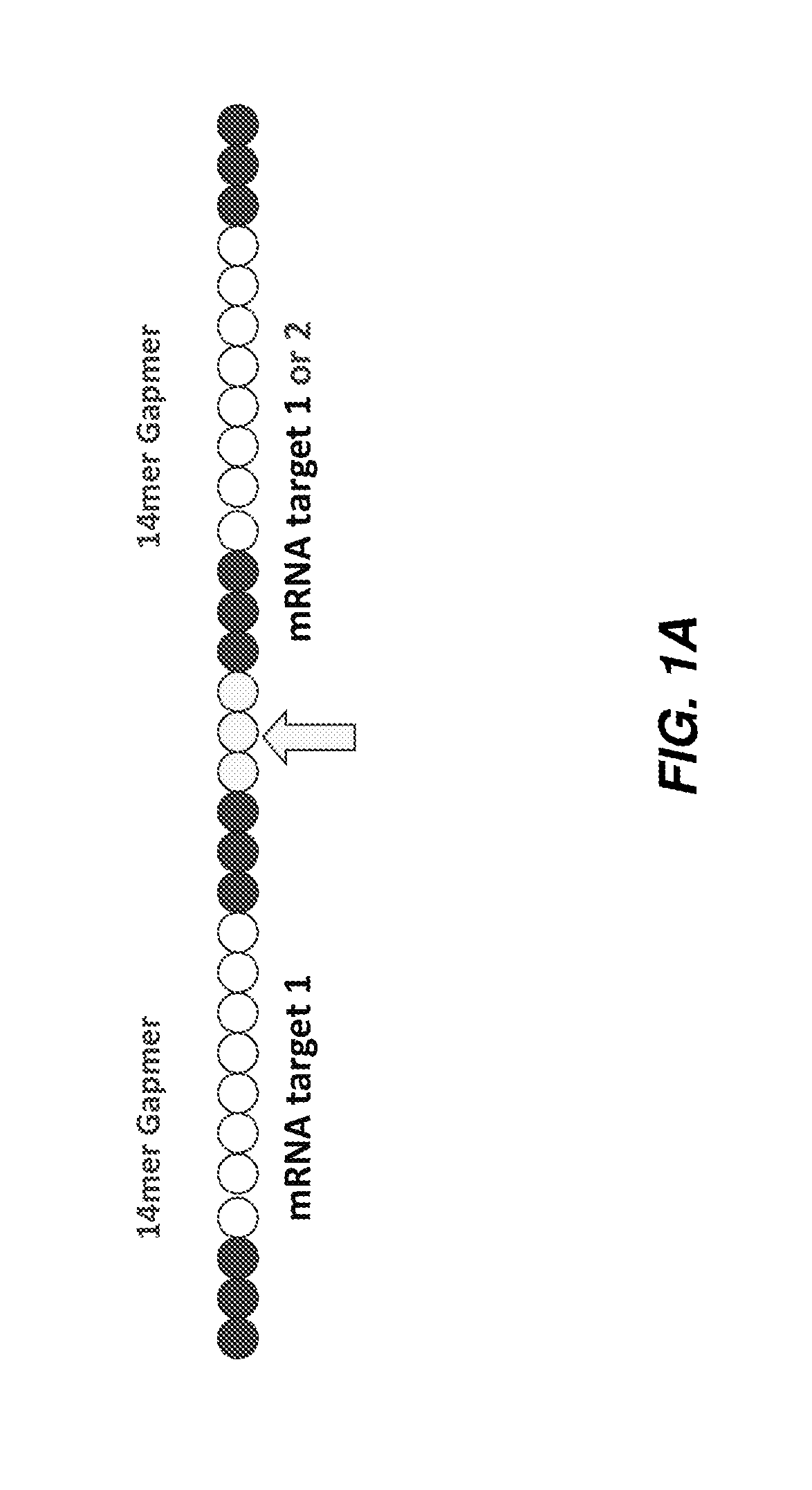
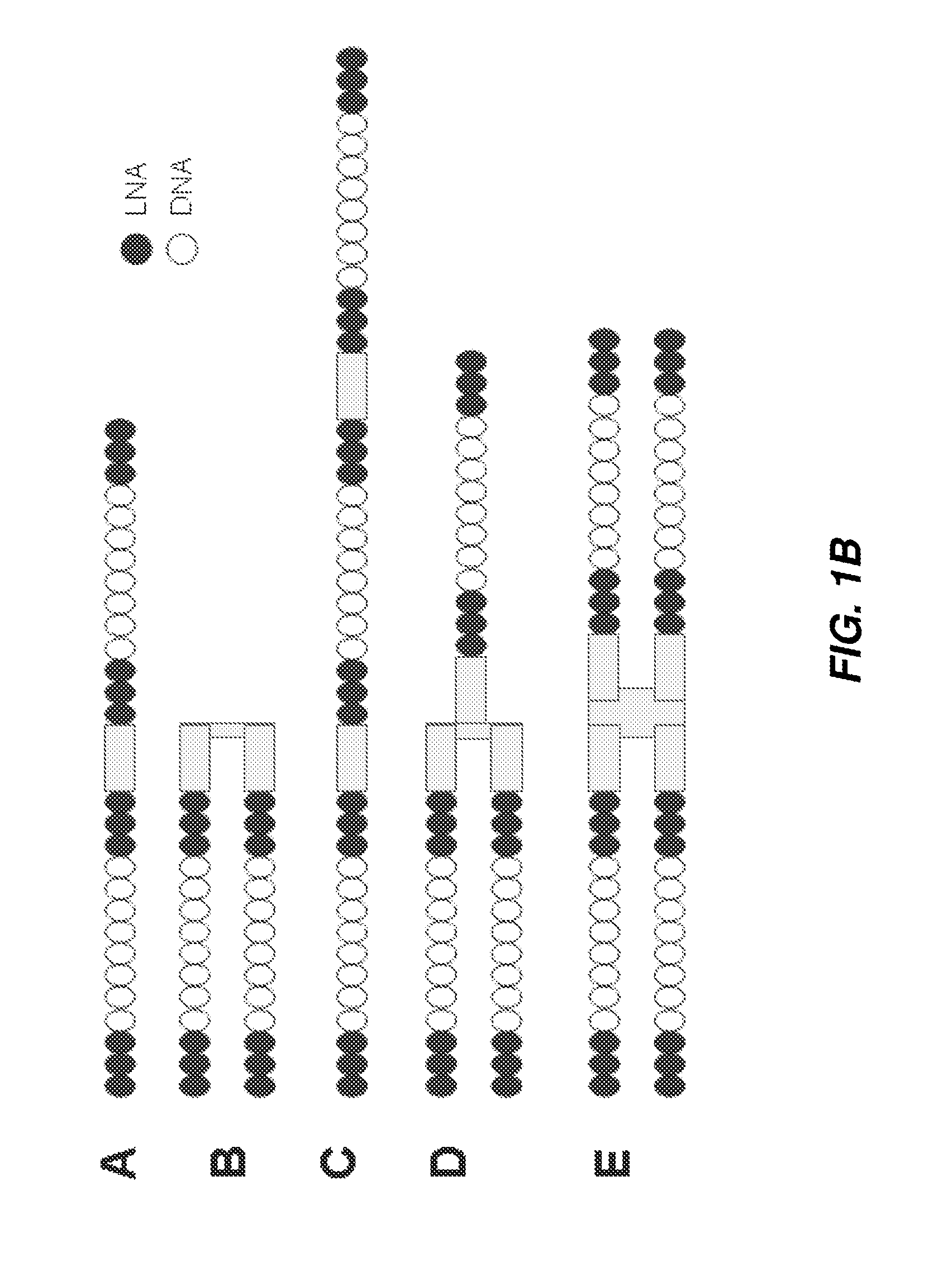
[0238]Antisense oligonucleotides against ApoC3, ApoB, Hif-1alpha, survivin and B2M were either selected using a series of bioinformatics filters and computational design algorithms or were derived from the literature. They were selected to be 13, 14 or more nucleotides in length and tested using one or multiple chemical modification design patterns (for example, 3LNAs-8DNAs-3LNAs). The list of all targeting oligonucleotide sequences is given in Table 1 and specific chemical modification patterns are explicitly specified when data is presented. Factors taken into account during the design include species homology, alignment to multiple human transcripts, off-target matches, SNPs, exon-exon boundaries, coverage of the transcript, and statistical models of efficacy and polyA regions. For species homology human, rat, mouse and macaque sequences were considered. For off-target matches, putative sequences were searched against the human transcriptome an...
example 2
Synthesis of Antisense Oligonucleotides
(A) General Procedure for Oligomer Synthesis
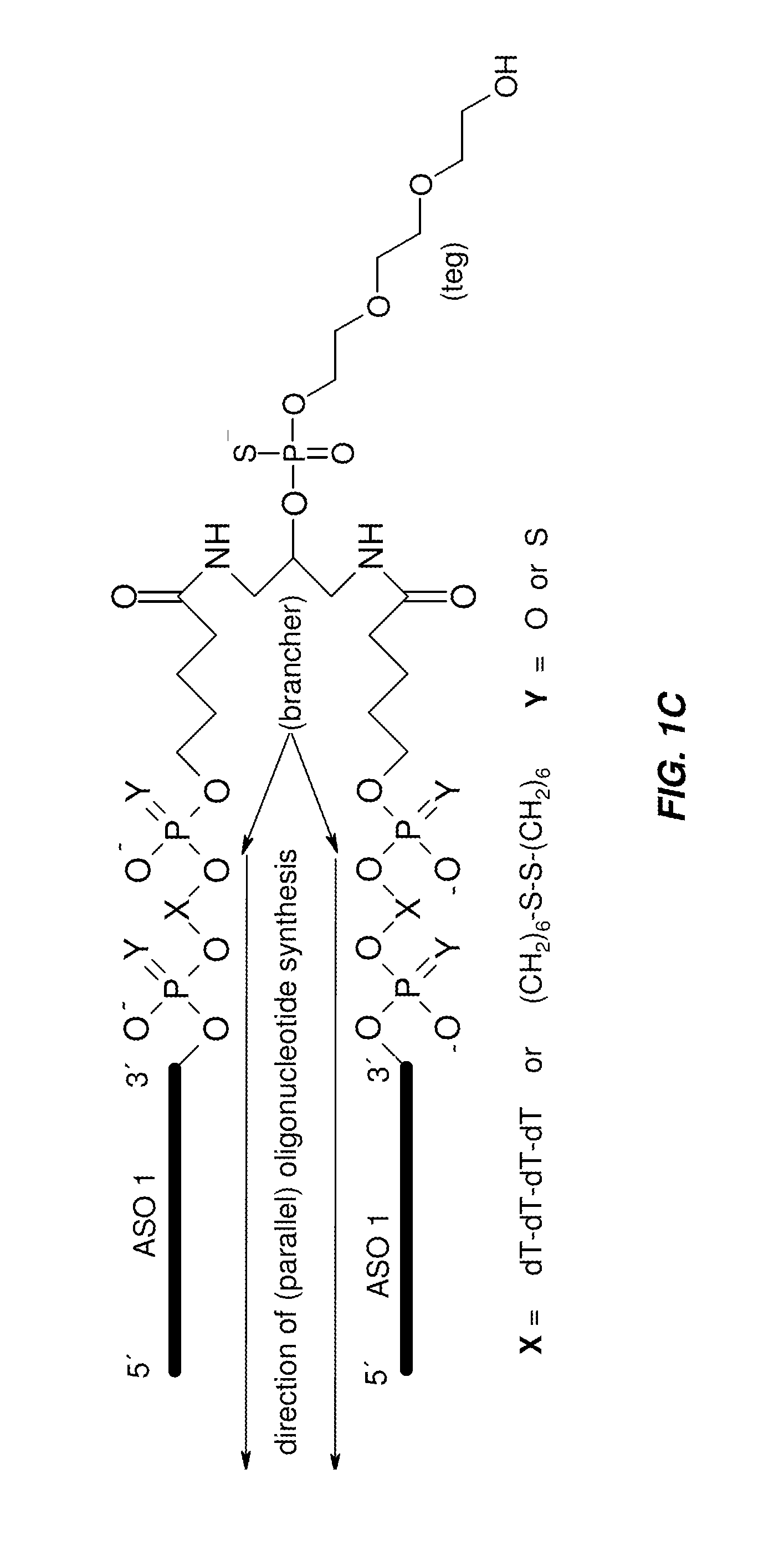
[0240]All oligonucleotides were synthesized using standard phosphoramidite protocols (Beaucage, S. L.; Caruthers, M. H. “Deoxynucleoside phosphoramidites—A new class of key intermediates for deoxypolynucleotide synthesis”. Tetrahedron Lett., 1981, 22:1859) on a MerMade 192 oligonucleotide synthesizer (BioAutomation) or Oligopilot 10 synthesizer (GE) at 200 to 1000 nmole scales employing standard CPG supports (BioSearch) or Glen UnySupport (Glen Research). The DNA, 2′-OMe, 2′-F, and G-clamp monomers were obtained from ChemGenes Corporation or Glen Research, and the LNA monomers were obtained from other commercially available sources. All phosphoramidites other than DNA were coupled with extended coupling times (e.g. 8 to 15 min for RNA, LNA, 2′-O-Methyl, 2′-Fluoro, 5-Propynyl and G-Clamps). After the synthesis, the oligonucleotides were cleaved from the support and deprotected using AMA (a 50:50 mixtur...
example 3
Dimer Stability in Plasma and Cleavage in Liver Homogenates
[0274]Stability measurements were performed using 4 different oligonucleotides (including dimers and the monomer, SEQ ID NOs: 1, 2, 3, 4).
[0275]Briefly, oligos were incubated in 95% plasma of mouse or monkey and in 5% liver homogenate at a concentration of 30 μM and at 37° C. Samples for measurement were taken after 0, 7, 24 and 48 h of incubation. Samples were subjected to a phenol / chloroform extraction and analyzed using LC-MS.
[0276]In detail, stock solutions with a final concentration of 600 μM and a final volume of 100 μl have been prepared of all oligonucleotides. Twelve pieces of approximately 50 mg of liver from CD1 mouse (female, Charles River) were added to individual Lysing matrix tubes. A calculated volume of 1×PBS to give a final concentration of 5% liver (W / W) was added to each of the twelve tubes. All samples were homogenized using a BioRad Fast prep System. The resulting homogenate solutions were combined to g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com