SiOx BARRIER FOR PHARMACEUTICAL PACKAGE AND COATING PROCESS
a technology of siox barrier and pharmaceutical package, which is applied in the direction of chemical vapor deposition coating, container/bottle contruction, rigid containers, etc., can solve the problems of insufficient stability, damage to stored materials, and non-uniform surface chemistry at the molecular level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0668]The following Examples are in part already disclosed in EP 2 251 455. In order to avoid unnecessary repetition, not all of the Examples in EP 2 251 455 A2 are repeated here, but explicit reference is herewith made to them.
Basic Protocols for Forming and Coating Syringe Barrels
[0669]The pharmaceutical packages or other vessels tested in the subsequent working examples were formed and coated according to the following exemplary protocols, except as otherwise indicated in individual examples. Particular parameter values given in the following basic protocols, for example the electric power and gaseous reactant or process gas flow, are typical values. When parameter values were changed in comparison to these typical values, this will be indicated in the subsequent working examples. The same applies to the type and composition of the gaseous reactant or process gas.
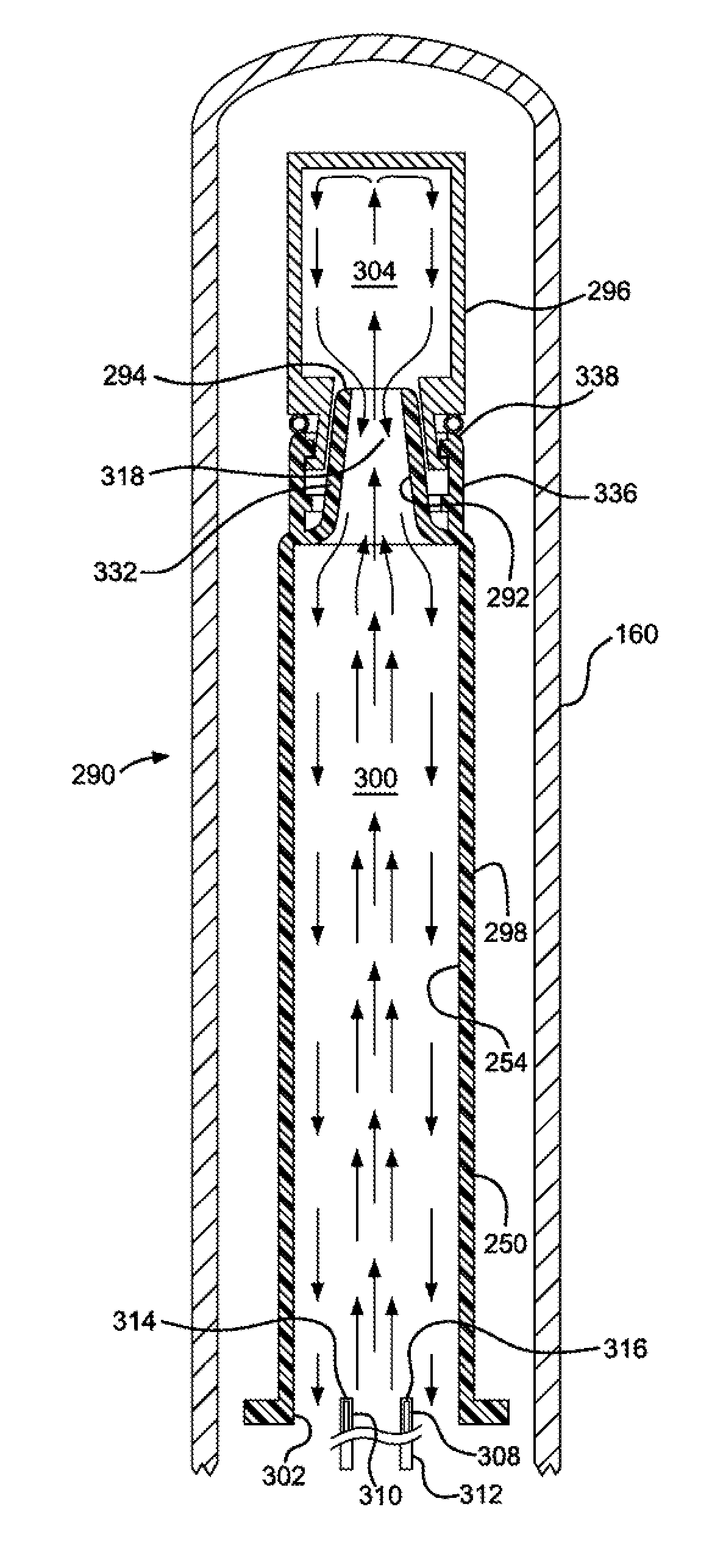
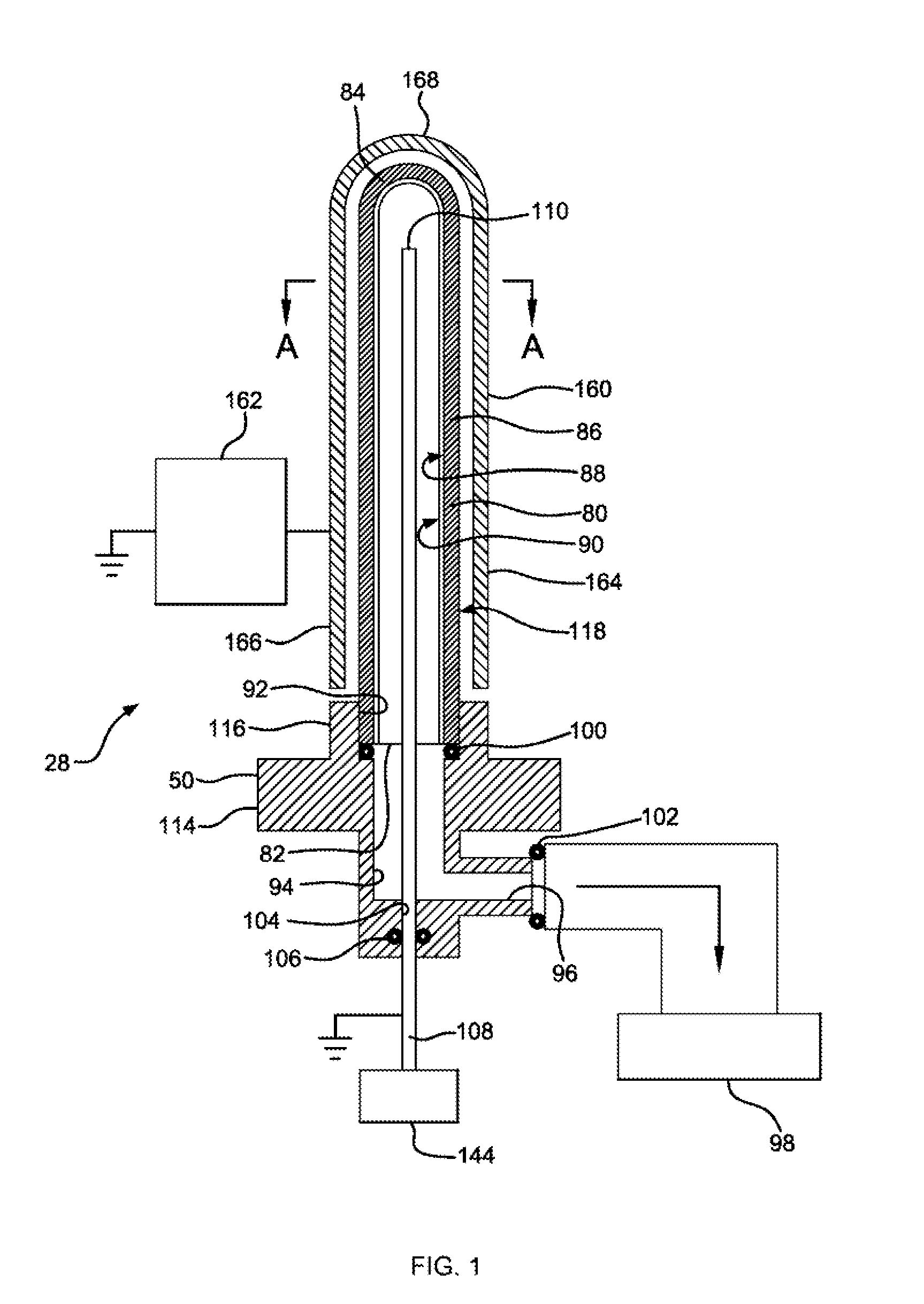
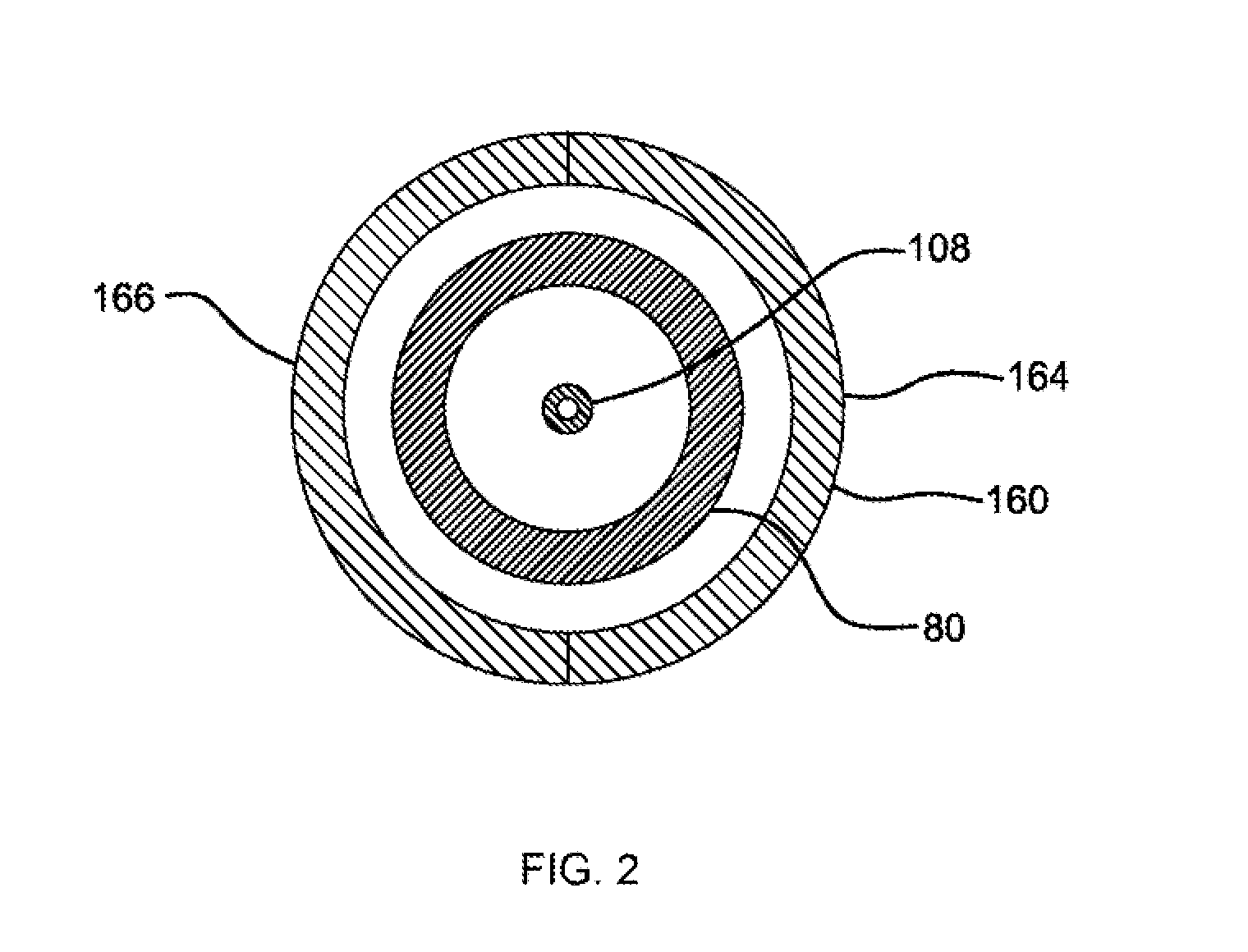
[0670]In some instances, the reference characters and Figures mentioned in the following protocols and additional deta...
examples 1-4
[0692]Syringe samples were produced as follows. A COC 8007 extended barrel syringe was produced according to the Protocol for Forming COC Syringe Barrel. An SiOx barrier coating or layer is applied to some of the syringes according to the Protocol for coating COC Syringe Barrel Interior with SiOx. A lubricity and / or pH protective coating or layer is applied to the SiOx coated syringes according to the Protocol for Coating COC Syringe Barrel Interior with OMCTS Lubricity Coating, modified as follows. The OMCTS was supplied from a vaporizer, due to its low volatility. Argon carrier gas was used. The process conditions were set to the following:[0693]OMCTS—3 sccm[0694]Argon gas—65 sccm[0695]Power—6 watts[0696]Time—10 seconds
[0697]Several syringes are then tested for lubricity using a Genesis Packaging Plunger Force Tester (Model SFT-01 Syringe Force Tester, manufactured by Genesis Machinery, Lionville, Pa.) according to the Protocol for Lubricity Testing. Both the initiation force and ...
examples 5-8
[0700]Syringe samples are produced as follows. A COC 8007 extended barrel syringe was produced according to the Protocol for Forming COC Syringe Barrel. An SiOx pH protective coating or layer is applied to the syringe barrels according to the Protocol for Coating COC Syringe Barrel Interior with SiOx. A lubricity and / or pH protective coating or layer is applied to the SiOx coated syringes according to the Protocol for Coating COC Syringe Barrel Interior with OMCTS, modified as follows. Argon carrier gas and oxygen are used where noted in Table 6. The process conditions are set to the following, or as indicated in Table 6:[0701]OMCTS—3 sccm (when used)[0702]Argon gas—7.8 sccm (when used)[0703]Oxygen 0.38 sccm (when used)[0704]Power—3 watts[0705]Power on time—10 seconds
[0706]The syringes of Examples 5 and 6 prepared under these conditions. The syringes of Example are 7 prepared under these conditions except without a lubricity and / or pH protective coating or layer, and the syringes of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com