An aqueous co2 absorbent comprising 2-amino-2-methyl-1-propanol and 3-aminopropanol or 2-amino-2-methyl-1-propanol and 4-aminobutanol
a technology of aqueous co2 and aqueous amine, which is applied in the direction of separation processes, hydrogen sulfides, sulfur compounds, etc., can solve the problems of reducing increasing the operating cost of co2 plants, and increasing so as to improve the oxidative degradation rate of amines and reduce the corrosive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
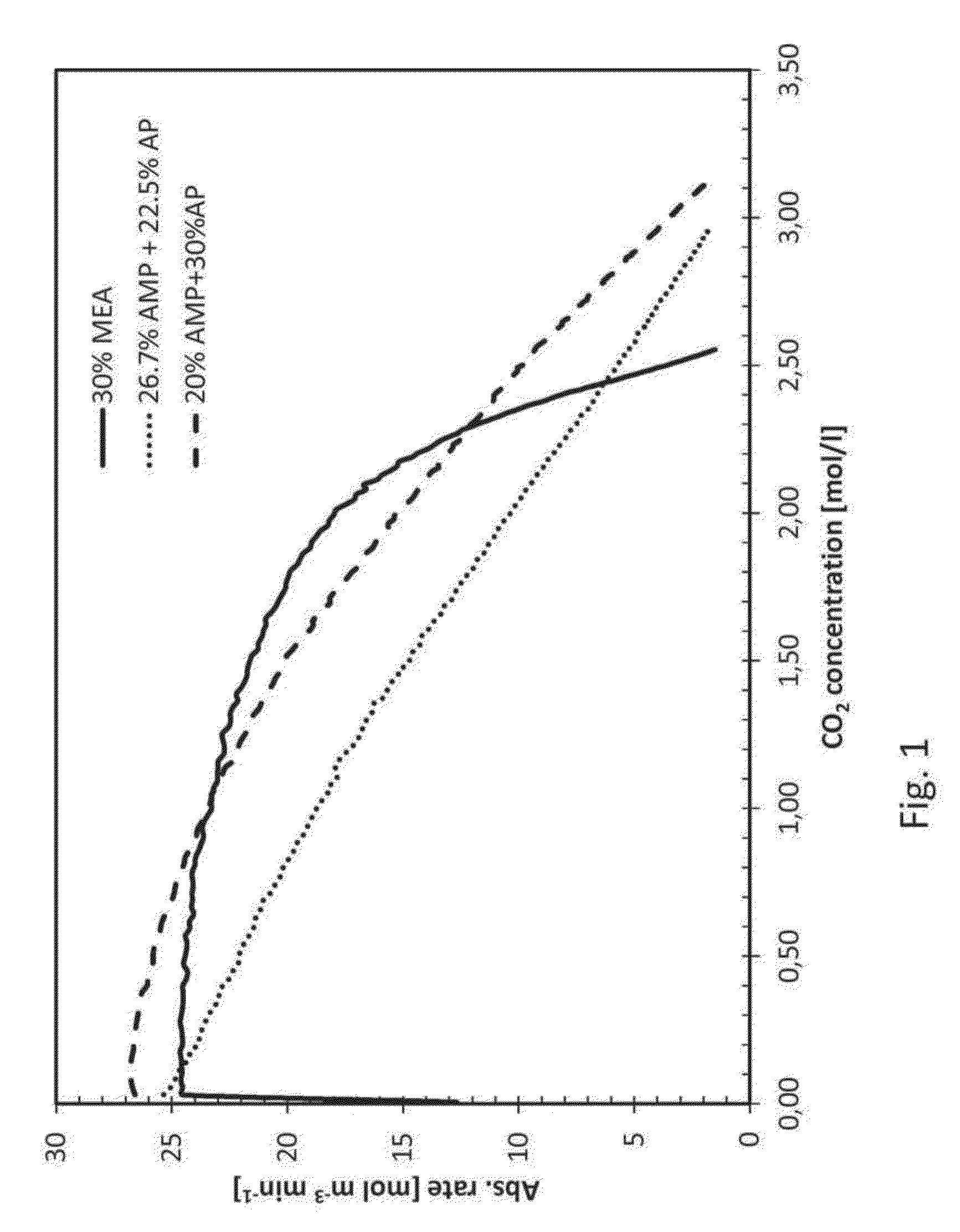
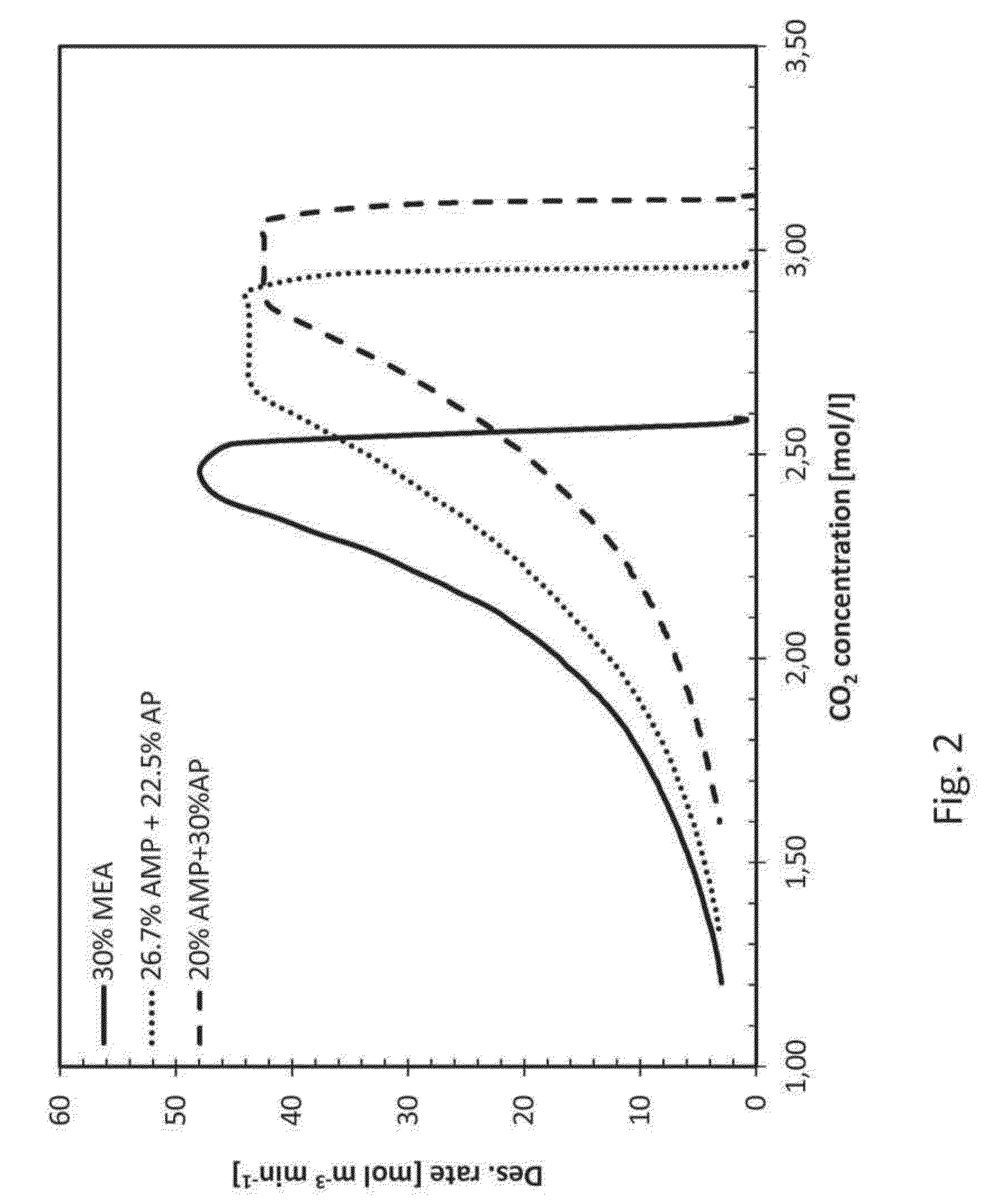
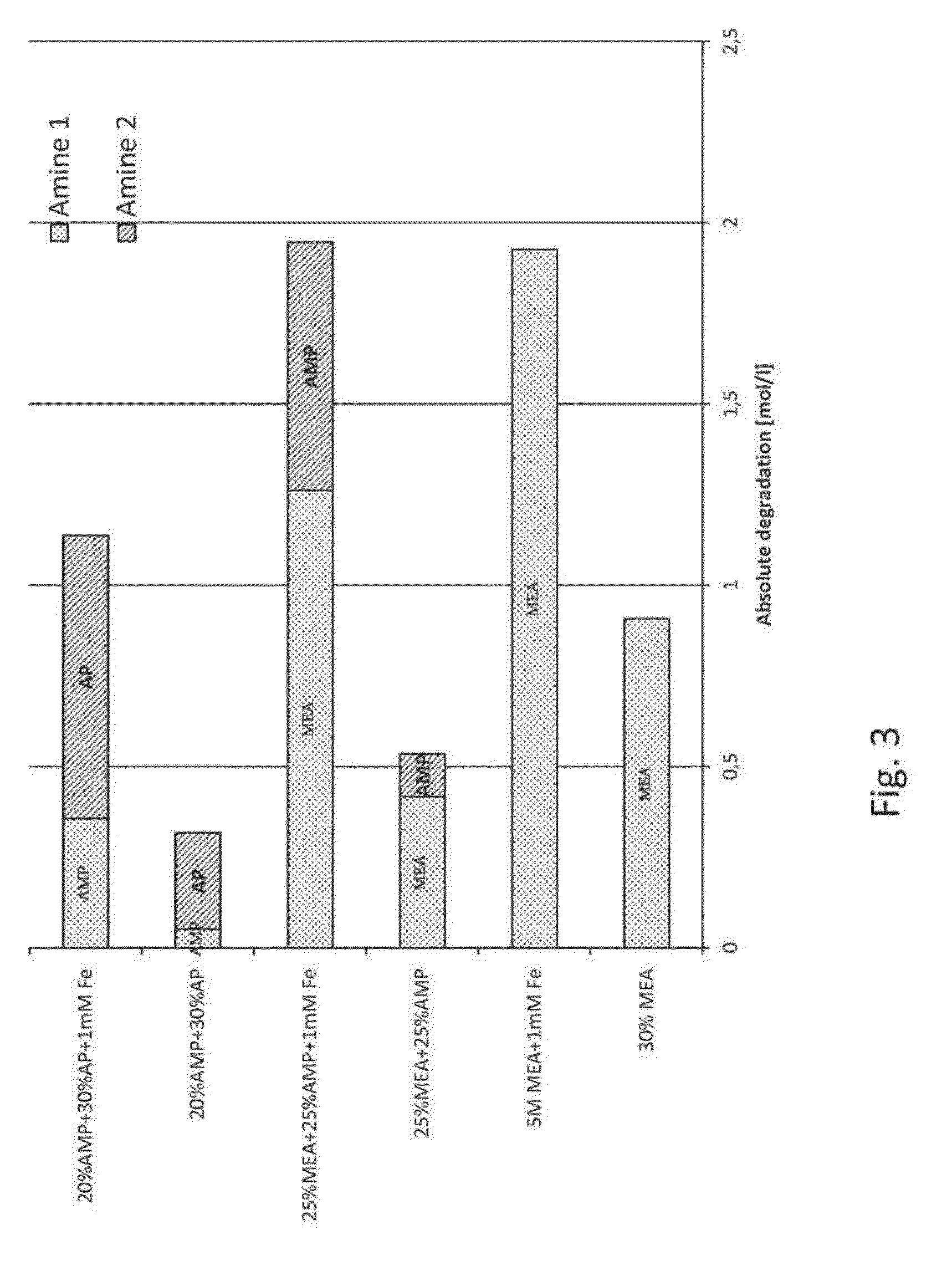
[0038]The present invention relates to an improved amine absorbent for CO2 capture and a method for capturing CO2 using the improved amine absorbent.
[0039]The invention is based on mixing two different primary amines having different reaction kinetics, one being a sterically hindered amine, namely 2-amino-2-methyl-1-propanol (AMP) and the other being a monoalkanolamine, namely 3-aminopropanol (AP), or 4-aminobutanol (AB).
[0040]AMP, being a sterically hindered amine, is known to have low energy requirement for regeneration of the absorbent but the slow reaction kinetics have a negative impact in the absorber as it requires a longer contact time between the CO2 containing gas and the absorbent in the absorber. As opposed to the reference amine, MEA, commonly used in 30 wt %, corresponding to a molar concentration of 5 mol / l, AMP cannot be used alone in higher concentrations than about 4 mol / l, corresponding to about 35% by weight, due to precipitation formed upon reaction with CO2. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com