Aqueous co2 absorbent comprising 2-amino-2-methyl-1-propanol and 3-aminopropanol or 2-amino-2-methyl-1-propanol and 4-aminobutanol
a co2 absorbent and amine technology, applied in the field of improved methods for capturing co2, can solve the problems of corrosive, heat demand is a major operating cost of the co2 plant, and the capture of cosub>2 is an energy-intensive process, so as to increase the oxidative degradation of amines, less corrosive, and less corrosive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038]The present invention relates to an improved amine absorbent for CO2 capture and a method for capturing CO2 using the improved amine absorbent.
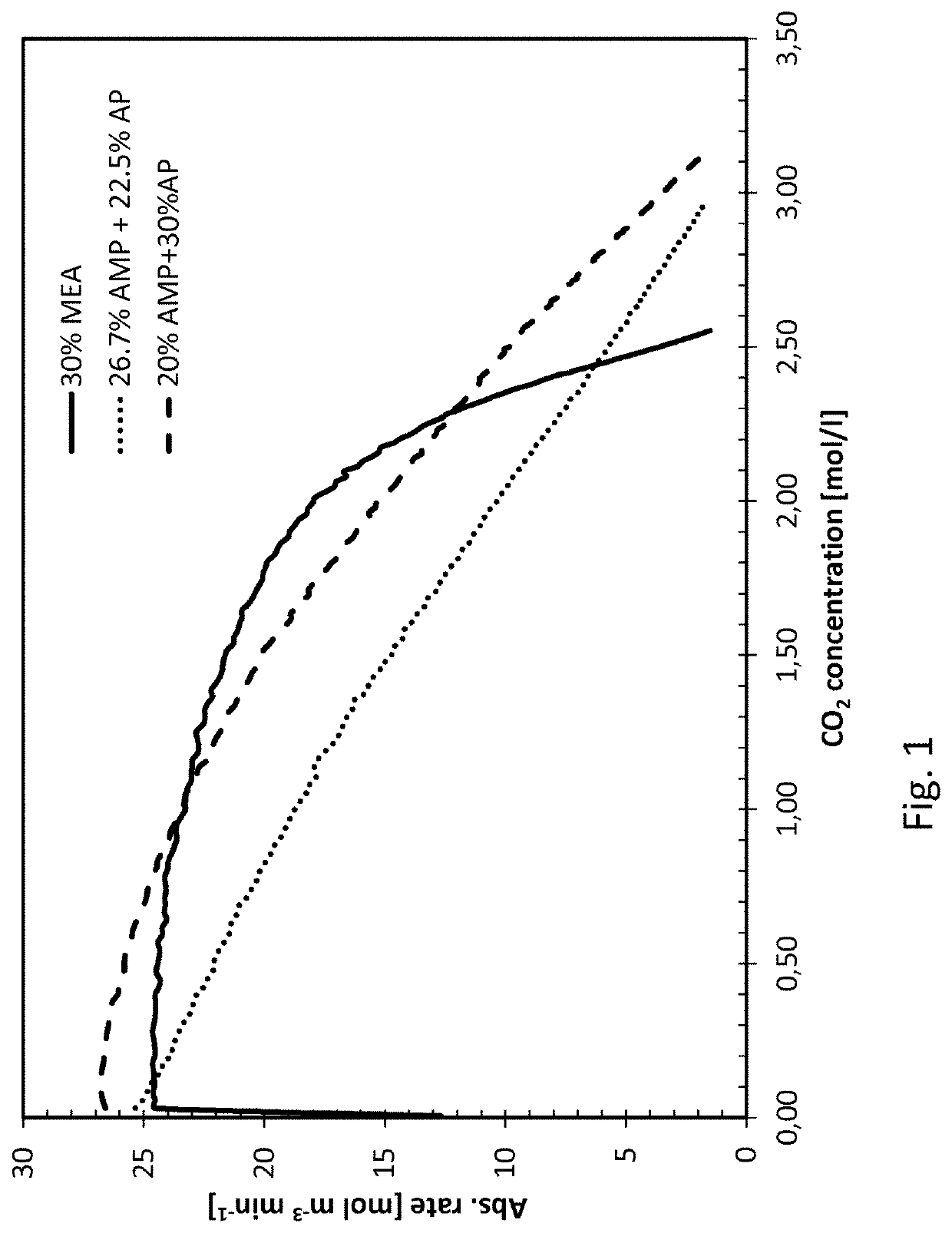
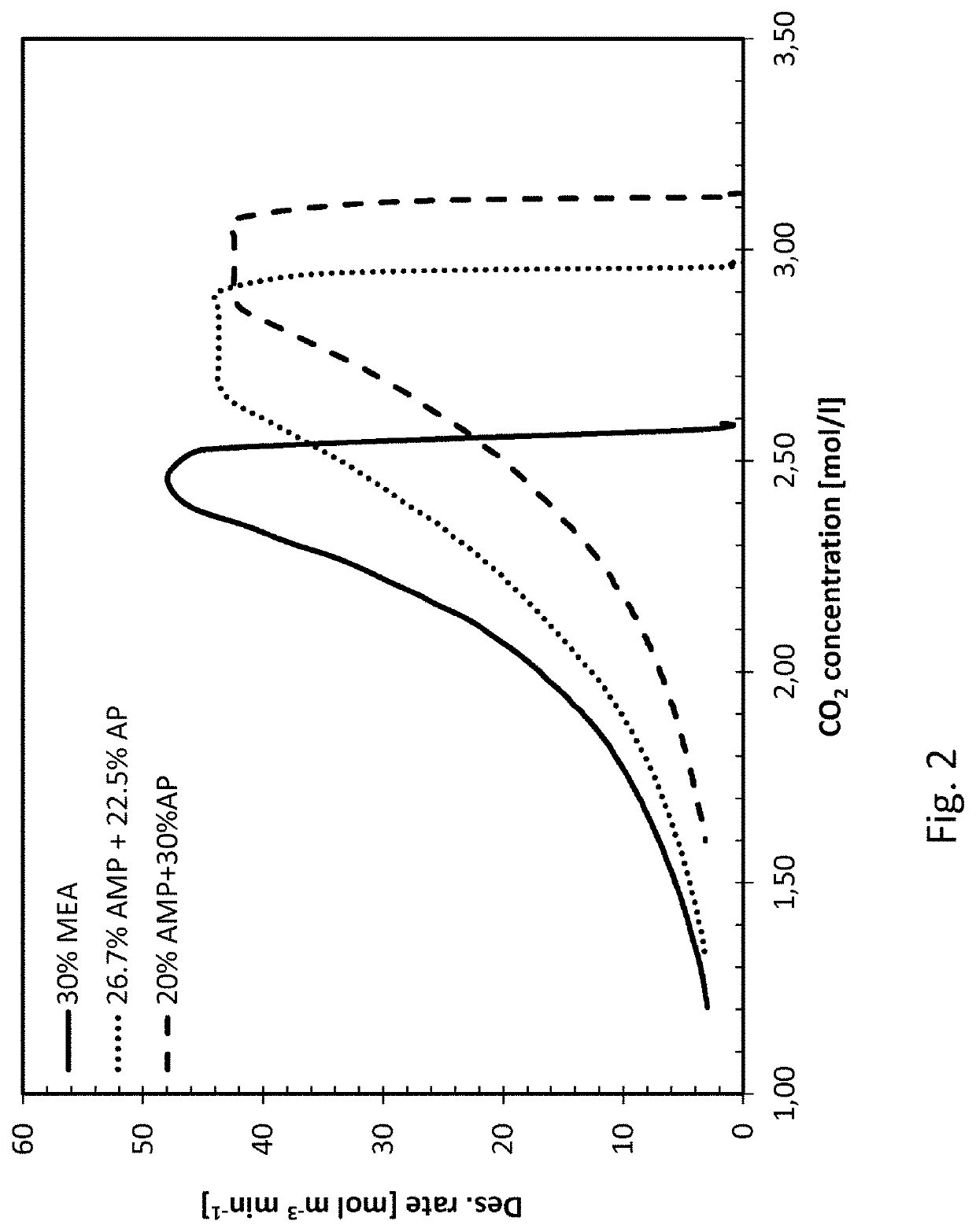
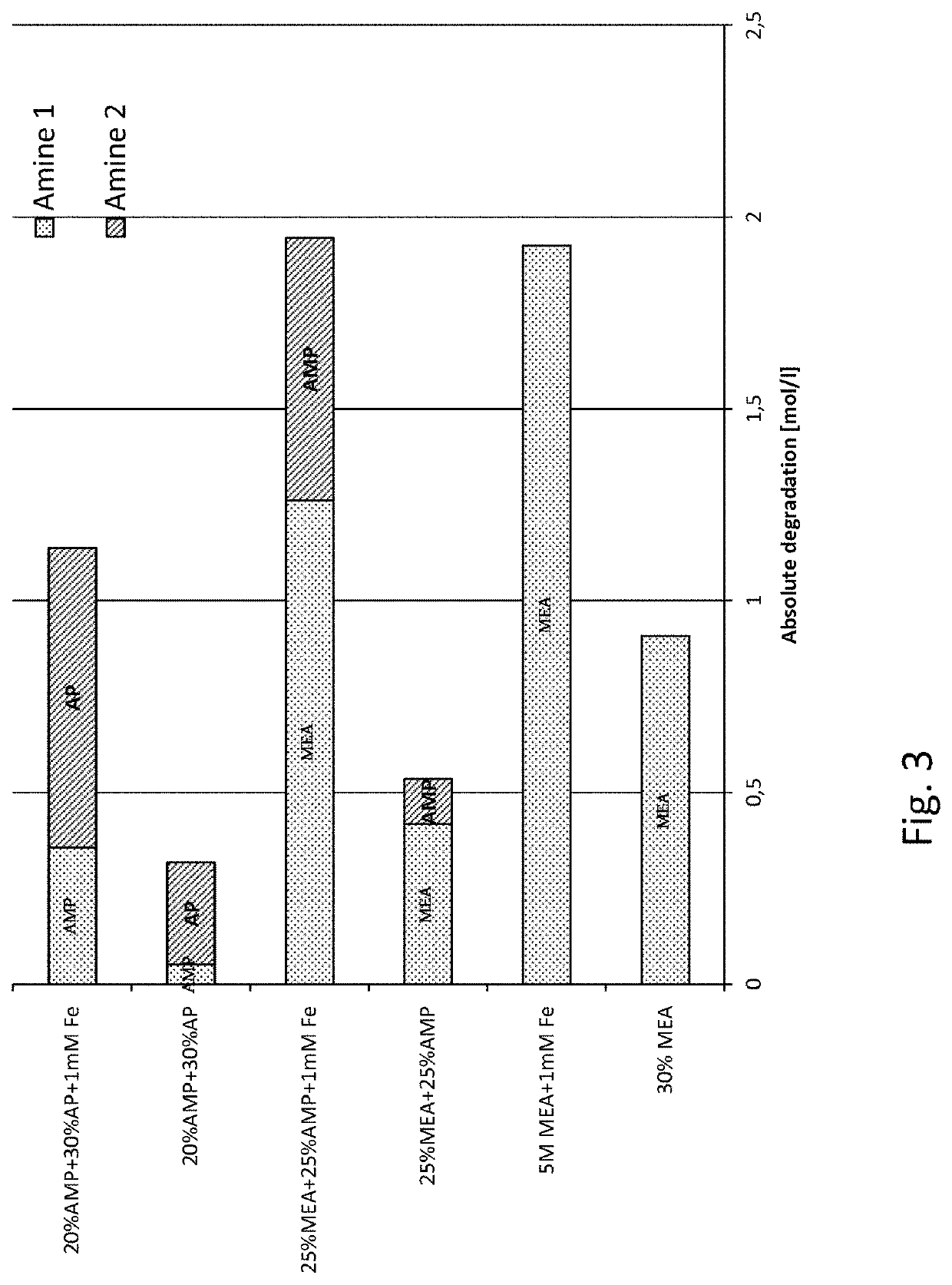
[0039]The invention is based on mixing two different primary amines having different reaction kinetics, one being a sterically hindered amine, namely 2-amino-2-methyl-1-propanol (AMP) and the other being a monoalkanolamine, namely 3-aminopropanol (AP), or 4-aminobutanol (AB).
[0040]AMP, being a sterically hindered amine, is known to have low energy requirement for regeneration of the absorbent but the slow reaction kinetics have a negative impact in the absorber as it requires a longer contact time between the CO2 containing gas and the absorbent in the absorber. As opposed to the reference amine, MEA, commonly used in 30 wt %, corresponding to a molar concentration of 5 mol / l, AMP cannot be used alone in higher concentrations than about 4 mol / l, corresponding to about 35% by weight, due to precipitation formed upon reaction with CO2. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com