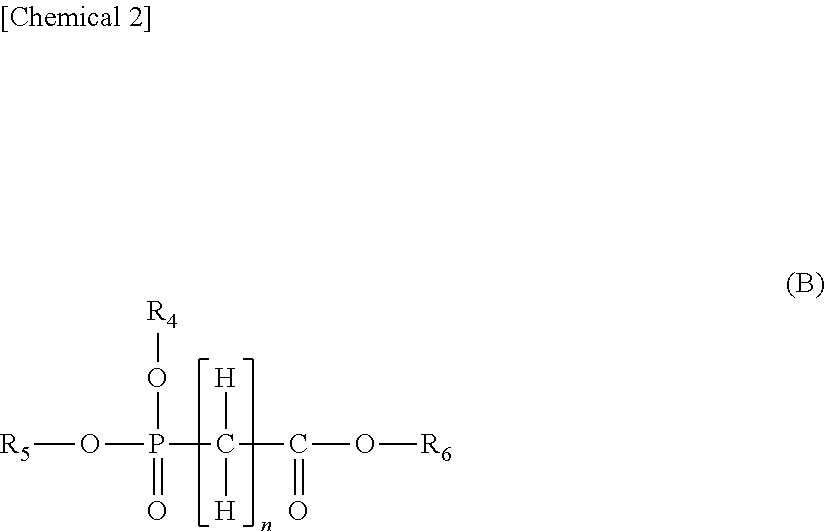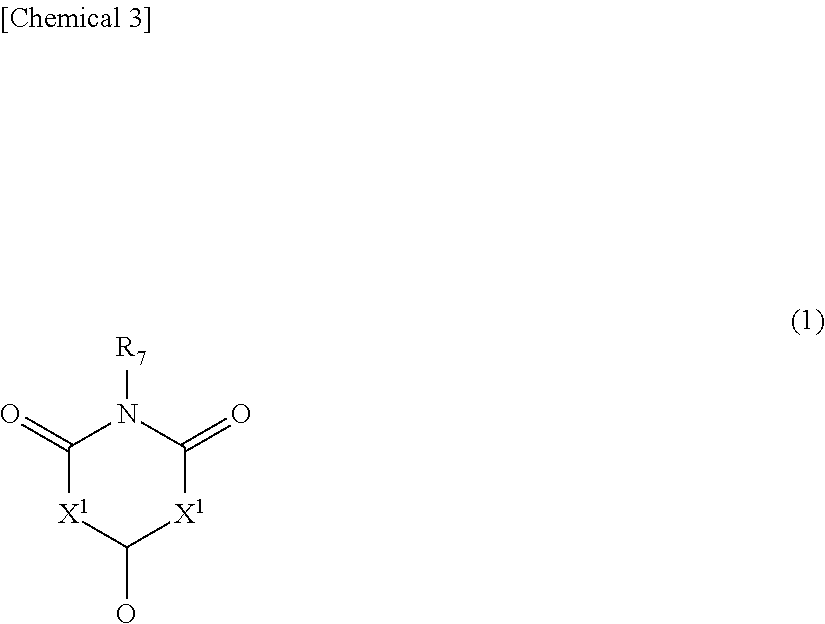Nonaqueous electrolyte solution and nonaqueous electrolyte battery using same
a technology of nonaqueous electrolyte and electrolyte battery, which is applied in the direction of batteries, cell components, electrochemical generators, etc., can solve the problems of increasing the internal resistance of the battery, reducing the battery capacity, and undergoing conventionally known capacity declines, so as to improve the high-temperature storage characteristics and cycle characteristics of the battery, and favorable overall balance of battery performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
I. First Embodiment
[0056]1. Nonaqueous Electrolyte Solution
[0057]1-1. Nonaqueous Electrolyte Solution of Present Invention
[0058]The nonaqueous electrolyte solution of the present invention contains a compound containing a structure represented by the following general formula (A), and at least one compound selected from the group consisting of a nitrile compound, an isocyanate compound, a difluorophosphate, a fluorosulfonate, a lithium bis(fluorosulfonyl)imide and a compound represented by the following general formula (B).
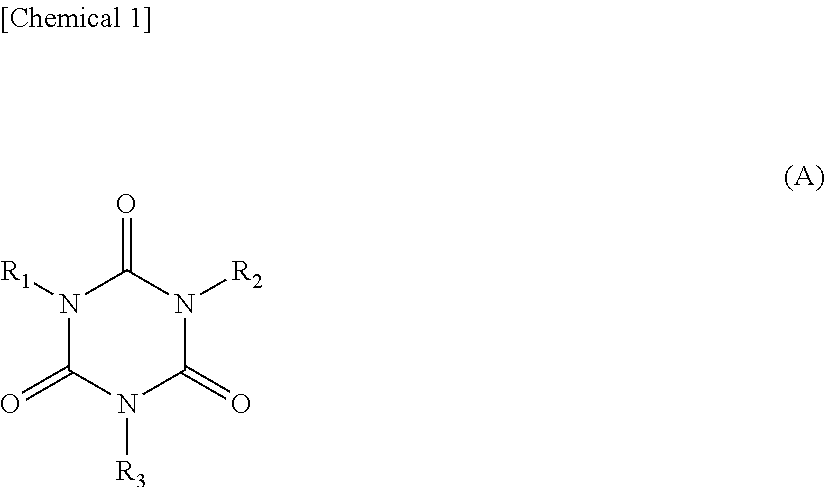
[0059]1-1-1. Compound Having Structure Represented by General Formula (A)
[0060]In formula (A), R1 to R3 may be mutually the same or different and are optionally substituted organic groups having 1 to 20 carbon atoms.
[0061]Here, an organic group represents a functional group composed of atoms selected from the group consisting of carbon atoms, hydrogen atoms, nitrogen atoms, oxygen atoms and halogen atoms. Specific examples thereof include an alkyl group, alkenyl g...
second embodiment
II. Second Embodiment
[0476]3. Nonaqueous Electrolyte Solution
[0477]The nonaqueous electrolyte solution of the present invention contains an electrolyte and a nonaqueous solvent that dissolves the electrolyte in the same manner as an ordinary nonaqueous electrolyte solution, and is characterized in that it contains a compound represented by the aforementioned general formula (1).
[0478]3-1. Compound Represented by General Formula (1)
[0479]The nonaqueous electrolyte solution of the present invention is characterized in that it contains a compound represented by the following general formula (1).
[0480]In the above formula, X1 and X2 independently represent NR8, NR8R9 or CR8R9, and R7 to R9 respectively and independently represent a hydrogen atom, fluorine atom, alkyl group, alkenyl group, alkynyl group or aryl group, and at least one of R7 to R9 represents a group having a cyano group and may be mutually the same or different. An alkyl group or alkenyl group is preferable, and an alkyl ...
examples
[0711]Although the following provides a more detailed explanation of the present invention by indicating examples and comparative examples, the present invention is not limited to these examples. Examples and comparative examples based on the first embodiment of the present invention are indicated below.
[0712]Compounds used in the present examples that contain a structure represented by general formula (A) are indicated below.
[0713]At least one type of compound selected from the group consisting of (1) nitrile compounds, isocyanate compounds, difluorophosphates, fluorosulfonates, lithium bis(fluorosulfonyl)imides and compound represented by general formula (B) used in the present examples are indicated below.
[0714](2) The cyclic carbonate compound having a fluorine atom used in the present examples is shown below.
[0715]Assistants (acid anhydride compounds: maleic anhydride, succinic anhydride, cyclic carbonate having a carbon-carbon unsaturated bond: vinylene carbonate) used in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| velocity | aaaaa | aaaaa |
| velocity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com