Apparatus and Method for Assessment of Interstitial Tissue
a technology of interstitial tissue and optical coherence tomography, which is applied in the field of apparatus and method for optical coherence tomography for assessment of interstitial tissue, can solve the problems of reducing the linearity of the scan, unable to accurately detect the interference pattern, and unable to achieve the effect of reducing the difficulty of each technique, so as to achieve minimal tissue disruption, high-resolution oct, and minimal invasive oct imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030]Generally, an apparatus including an optical probe capable of taking an OCT image is inserted into interstitial tissue. The optical probe is manually moved (e.g., by a technician or a robot). When the optical probe is moved more than approximately 5 microns from a reference point (e.g., a guidance needle or a handle), an OCT reflectivity profile or A-line is taken. Each OCT A-line is analyzed by a data processor to determine whether or not it is a repeat of the previous A-line. Repeated A-lines are discarded. The data process presents an aggregate OCT image of the interstitial tissue based on each non-discarded OCT A-line.
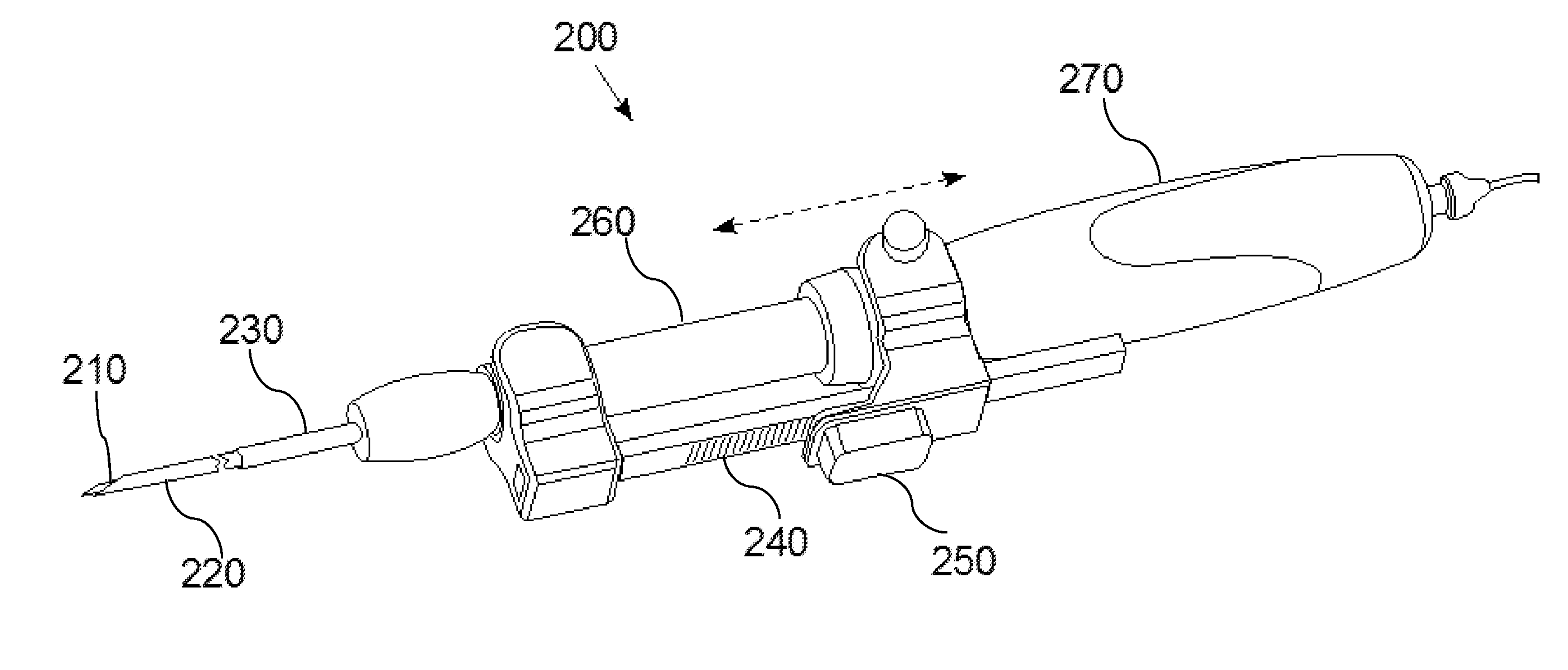
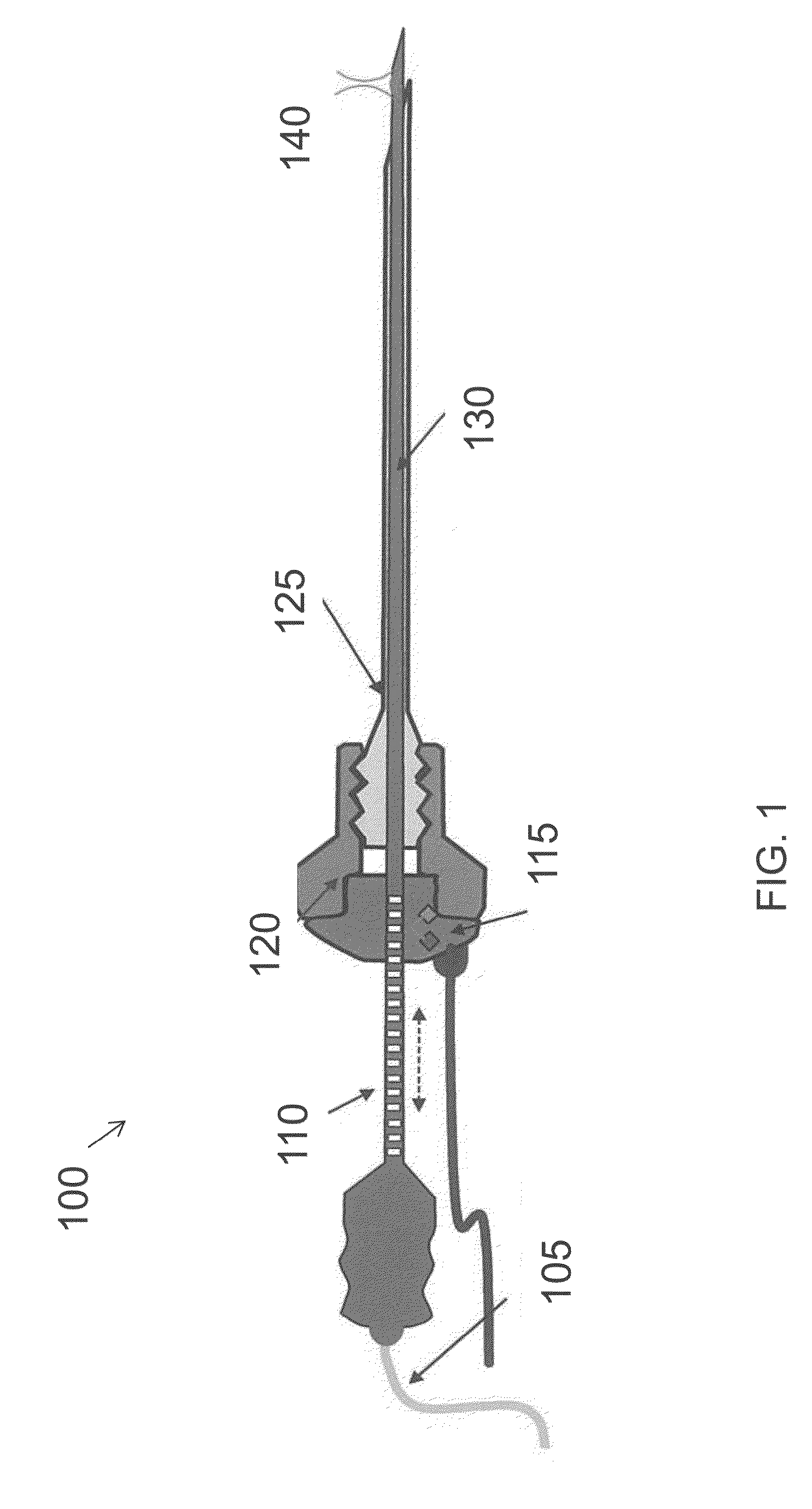
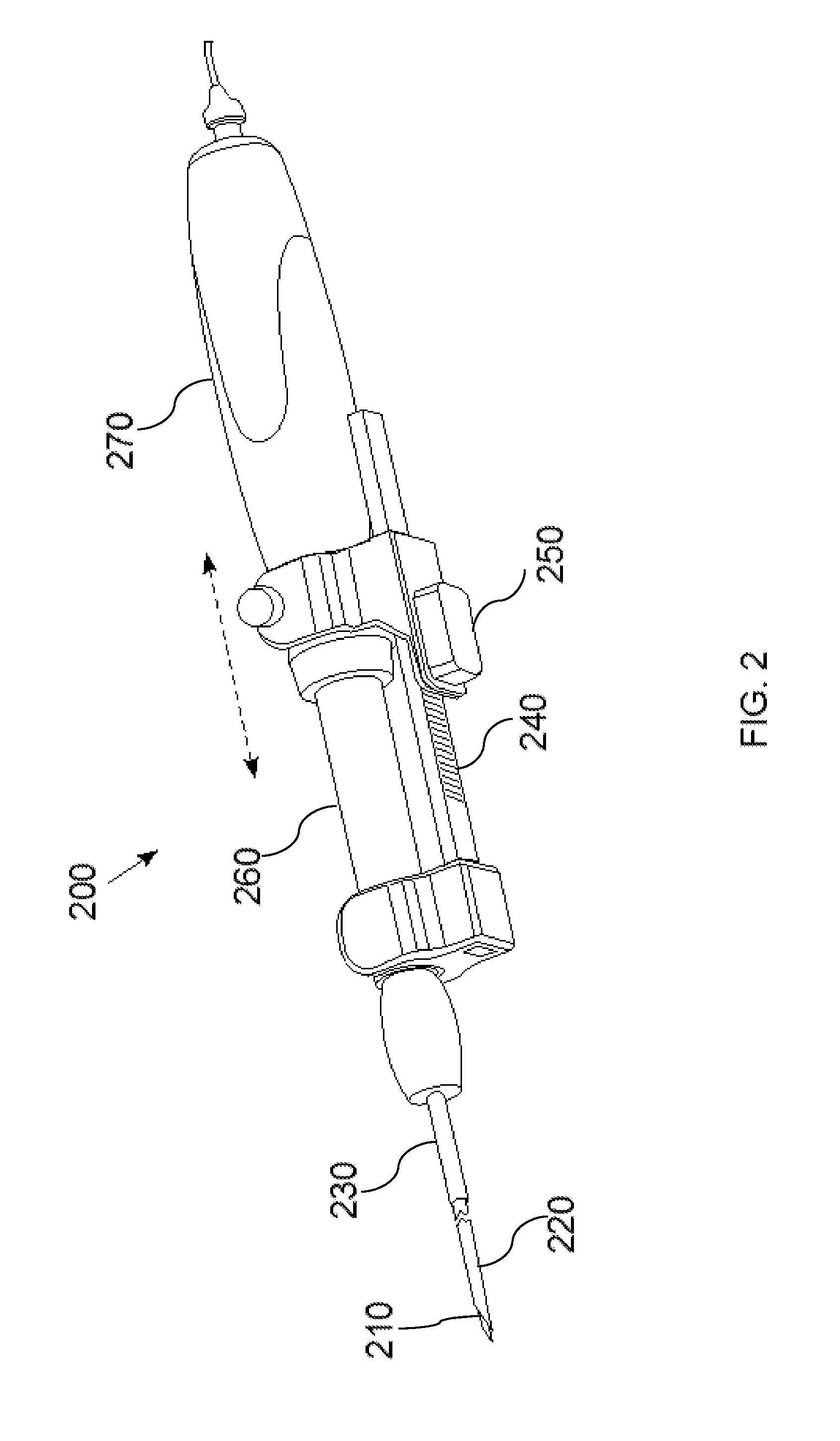
[0031]FIG. 1 is a diagram of a hand-held optical imaging device 100, according to an illustrative embodiment of the invention. The hand-held optical imaging device 100 includes an optical fiber 105, an optical scale 110, a position sensor 115 (e.g., optical encoder), a connector 120 (e.g., male luerlock connector), a guidance needle 125, and an optical probe ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com