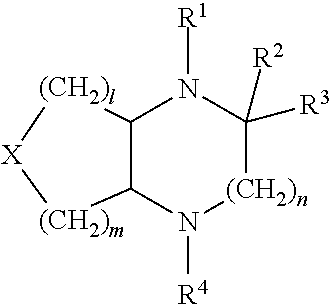
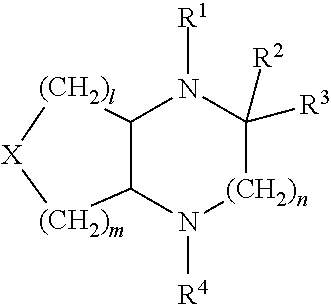
Heterocyclic compounds for treating or preventing disorers caused by reduced neurotransmission of serotonin, norephnephrine or dopamine
a heterocyclic compound and neurotransmission-reducing technology, applied in the field of new heterocyclic compounds, can solve the problems of failing to exert sufficient therapeutic effects on approximately 30% of patients, and achieve the effects of short time, sufficient therapeutic effects, and wide therapeutic spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Production of cis-3,3-dimethyloctahydrocyclopentapyrazin-2-one
Relative Configuration
[0208]
[0209]90% / o acetone cyanohydrin (9.79 g, 104 mmol) was added to an aqueous (100 mL) solution of cis-cyclopentane-1,2-diamine (9.88 g, 98.6 mmol) at room temperature, and the mixture was stirred under reflux for 16 hours. The solvent was removed from the reaction mixture under reduced pressure, followed by azeotropy with ethanol. The obtained residue was purified by silica gel column chromatography (methylene chloride / methanol=1 / 10) to obtain cis-3,3-dimethyloctahydrocyclopentapyrazin-2-one (5.00 g, 30%) in a white powder form.
[0210]1H-NMR(CDC3) δppm: 1.20 (1H, brs),1.34 (3H, s),1.39 (3H, s),1.40-2.20 (6H, m), 3.50-3.70 (2H, m),5.89 (1H, brs).
[0211]Compounds of Reference Examples 2 to 12 shown below were produced in the same way as in Reference Example 1 using appropriate starting materials.
reference example 2
Trans-3,3-dimethyloctahydrocyclopentapyrazin-2-one
Relative Configuration
[0212]
[0213]1H-NMR (CDCl3) δppm: 1.26-1.55 (9H, m), 1.75-2.00 (4H, m), 2.85-3.02 (1H, m),3.05-3.20 (1H, m),6.02 (1H, brs).
reference example 3
Cis-3,3-dimethylhexahydrofuro[3,4-b]pyrazin-2-one
Relative Configuration
[0214]
[0215]1H-NMR (CDCl3) δppm: 1.37 (3H, s), 1.40 (3H, s), 1.50-1.85 (1H, br),3.73-4.10 (6H, m),6.02-6.22 (1H, br).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com