Transoral dosage forms comprising sufentanil and naloxone
a transoral and sufentanil technology, applied in the direction of biocide, drug composition, aerosol delivery, etc., can solve the problems of reduced analgesia, low bioavailability of free base, and limited sufentanil solution that can penetrate the oral mucosal tissues, so as to improve the safety margin, less ventilory depression, and the effect of improving the safety margin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sufentanil and Fentanyl Solutions
[0080]Excess sufentanil base and fentanyl base were added to pH 5 citric acid buffers made up in deionized water to make stock solutions of sufentanil citrate and fentanyl citrate. The stock solutions were equilibrated overnight @ 37° C., and the solution concentrations of drug were estimated to be at saturation, e.g. sufentanil (4.76 mg / ml) and fentanyl (17.35 mg / ml) @ 37° C. The stock solutions were iteratively titrated to the desired test pH of pH 5.0 with 0.1M of citric acid solution prior to being used experimentally; pH was not further measured during the permeation studies in the Examples below.
example 2
In Vitro Evaluation of Permeability of Sufentanil and Fentanyl Through Pig Buccal Mucosal Tissue
[0081]In-vitro permeation buccal flux studies were conducted with fresh pig buccal tissue. Prior to the in vitro buccal flux experiment, the buccal tissue was cleaned in tap water at room temperature, and the muscle tissue was removed by surgical knife and scissor. The buccal tissue was cut into 1-inch circular specifimens, being careful to exclude any damaged tissue areas.
[0082]Next, a pre-cut buccal tissue specimen was positioned on the top edge of the receptor side of a modified Franz cell with the basolateral side of the buccal tissue facing the receptor chamber. The donor side of the Franz Cell was securely positioned over the skin / system assembly, and fitted with a plastic cap to avoid evaporation of the donor solution. The receptor chamber was filled with citrate buffer at pH 5.0 and was constantly stirred, @ approximately 400 rpm, with the use of a Teflon coated magnetic spin bar....
example 3
In Vitro Evaluation of Permeability of Sufentanil and Fentanyl Through Pig Buccal Mucosal Tissue
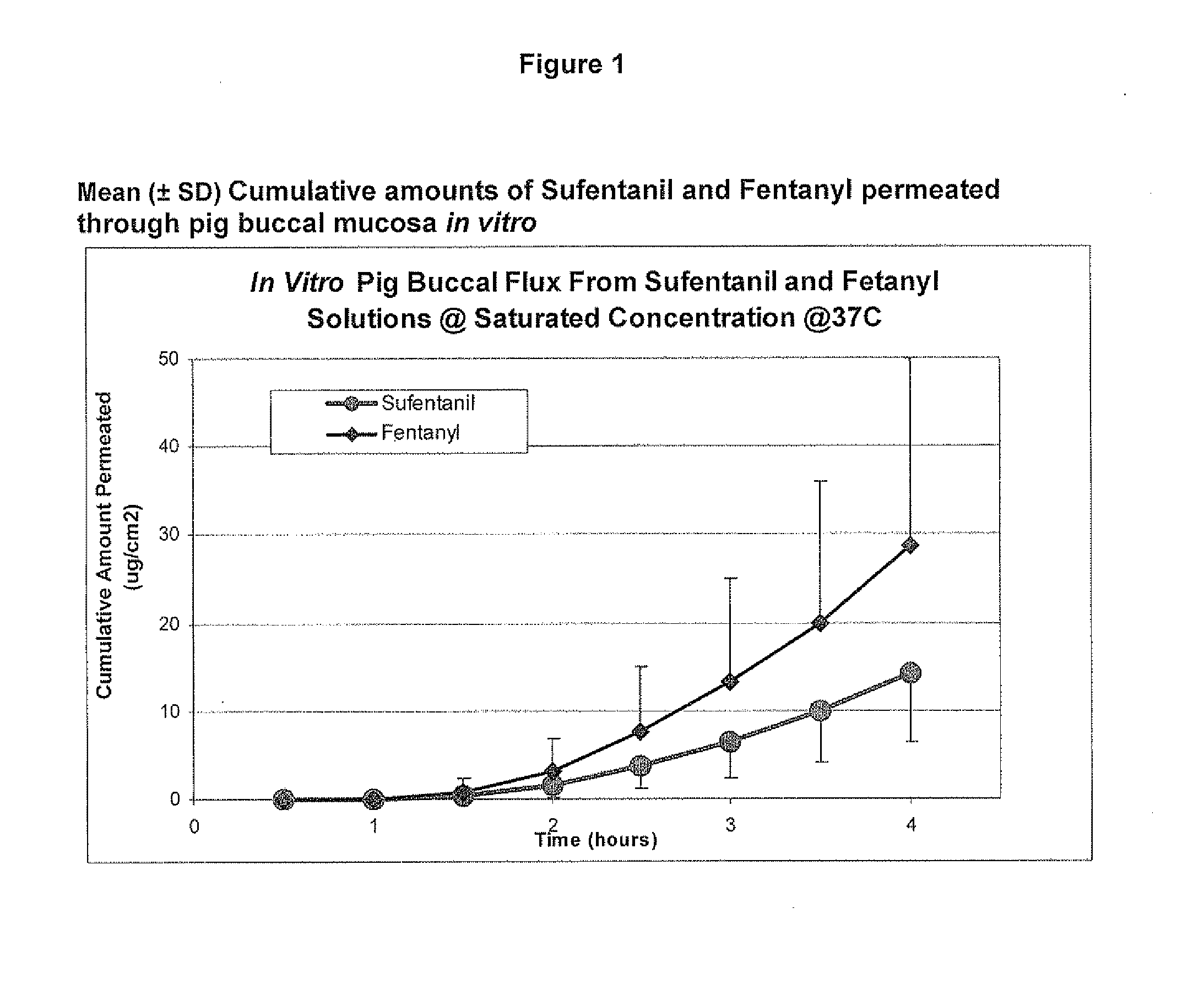
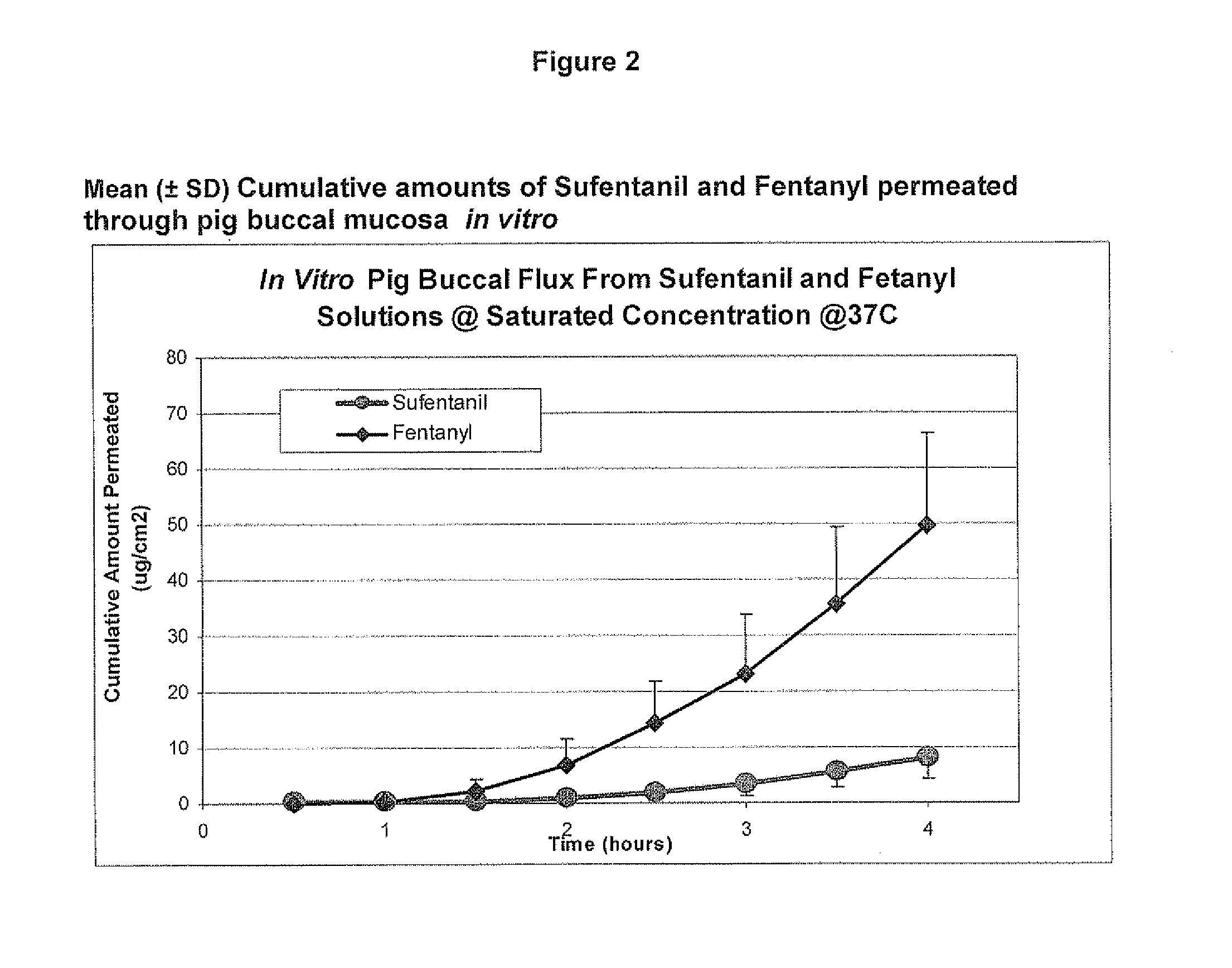
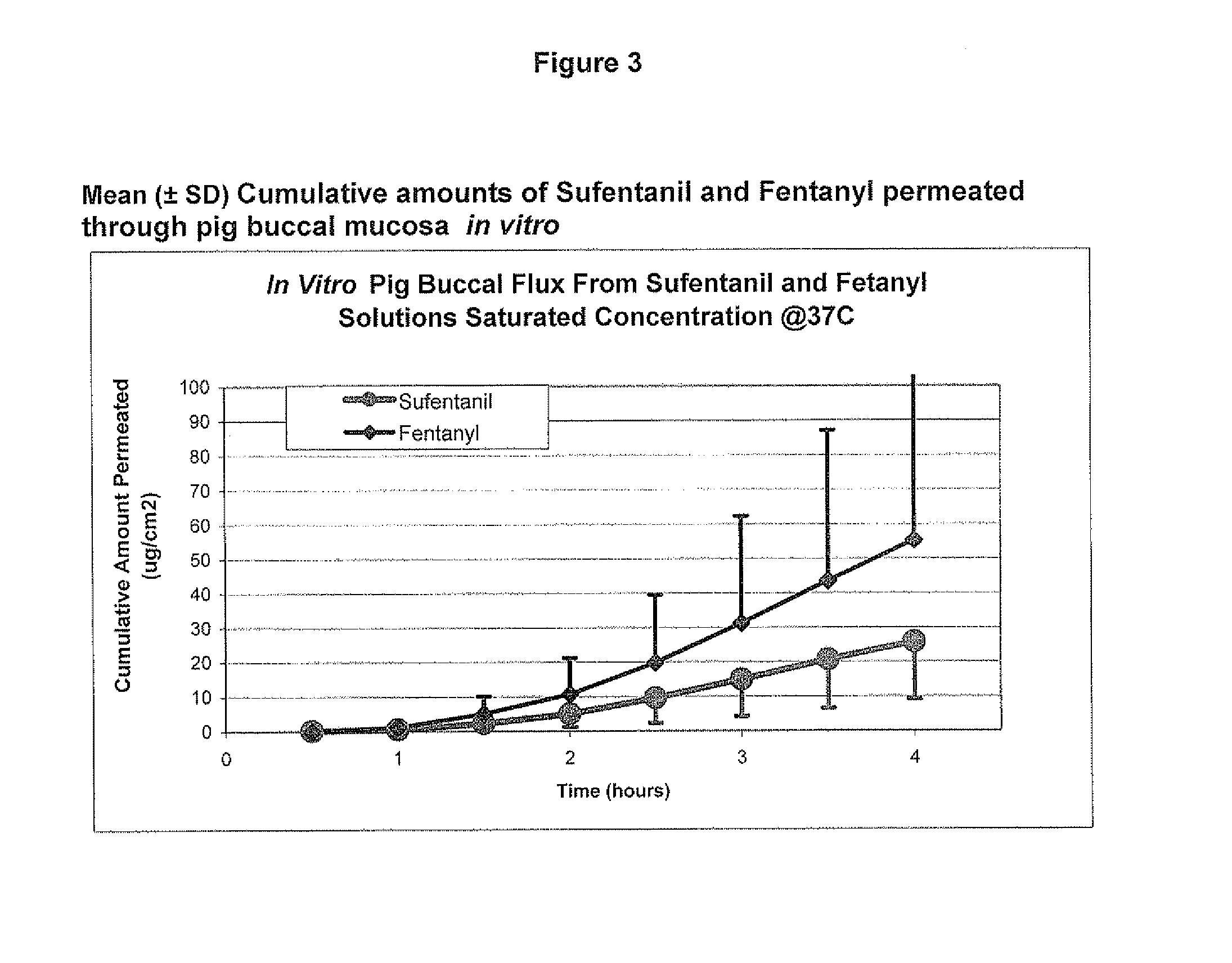
[0086]Example 2 was repeated, using different porcine buccal tissue samples. The results are shown in Table 2 and FIG. 2. Three test cells were averaged at each time point to arrive at the reported values.
TABLE 2Mean (±SD) Cumulative Amount Permeated through pig buccalmucosa (ug / cm2) @ 37° C.Drugs0.5 (h)1 (h)1.5 (h)2 (h)2.5 (h)3 (h)3.5 (h)4 (h)Sufentanil0.2 ± 0.30.2 ± 0.30.3 ± 0.30.8 ± 0.5 1.9 ± 1.23.4 ± 2.05.6 ± 2.98.3 ± 3.9Fentanyl0.0 ± 0.00.4 ± 0.72.1 ± 2.46.8 ± 4.714.4 ± 7.523.2 ± 10.535.6 ± 13.749.6 ± 16.6
PUM
| Property | Measurement | Unit |
|---|---|---|
| equivalent weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com