Multi-tailed lipids and uses thereof
a technology of lipids and lipids, applied in biochemistry apparatus and processes, organic chemistry, fermentation, etc., can solve the problems of slow slowed development of genetic drugs, and difficult sirna delivery efficiency,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Compounds
[0345]Recent high-throughput screens of lipid-like compounds indicate that structures with multiple short alkyl tails stemming from a polyamine core are able to facilitate efficient gene silencing (3, 16, 17). To synthesize pure multi-tailed compounds with varying polyamine head group architectures, a rapid and efficient two-step solvent free synthetic route was developed that requires neither protection / deprotection steps nor the use of costly and time-consuming chromatographic purification (see, e.g., Schemes 1 and 2). The first step involves a quantitative Michael addition between a primary amine 2 or secondary diamine 5 and propargyl acrylate 1. The resulting bis-alkyne modified amines 3 and 6 are subjected to a thiol-yne “click” photoaddition with decanethiol 4 in the presence of a catalyst (e.g., a photocatalyst, such as a photoinitiator). The thiol-yne reaction is rapid and efficient and yields pure four-tailed lipid products in only about 180 seco...
example 2
Biological Assays
General Methods
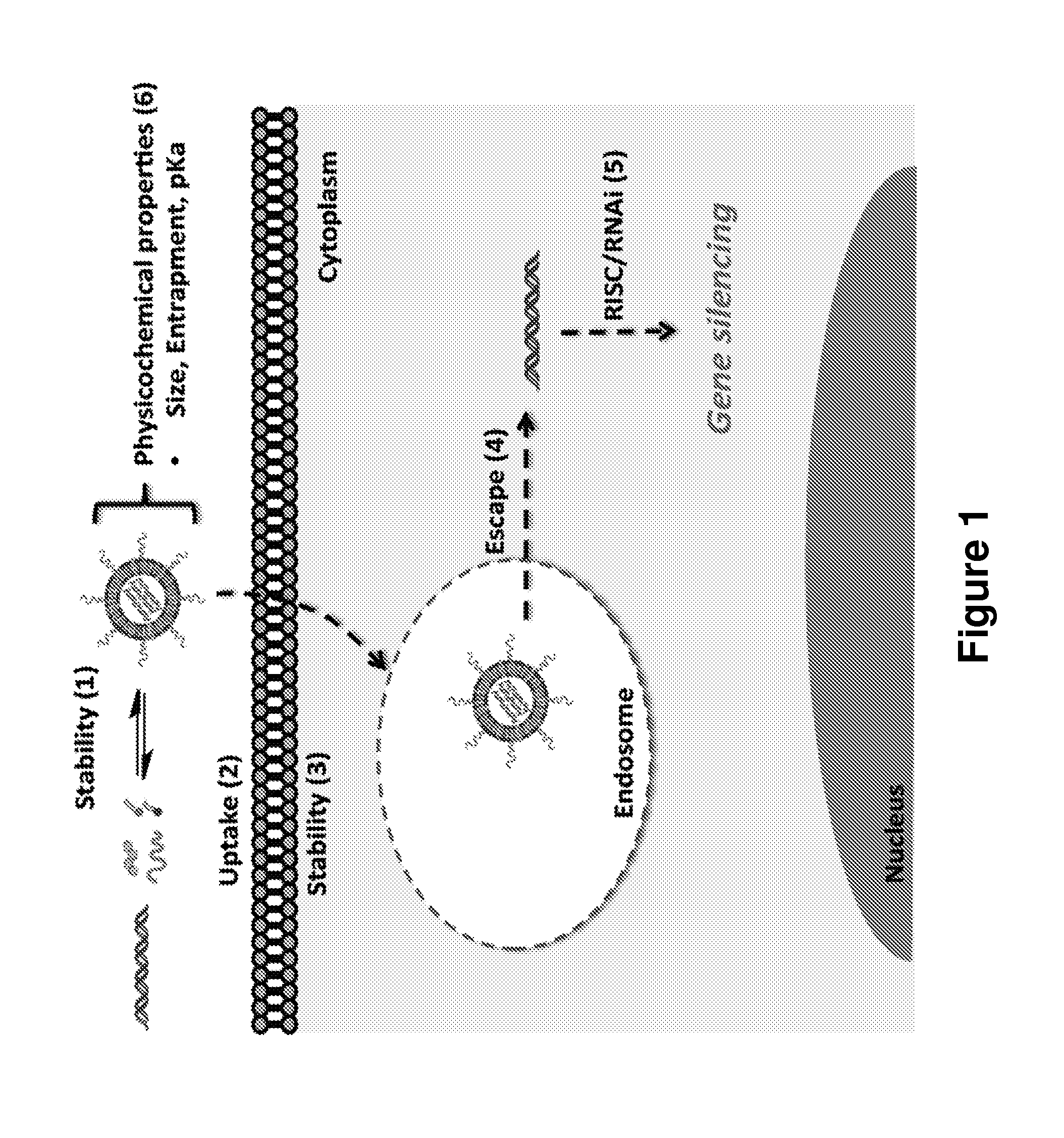
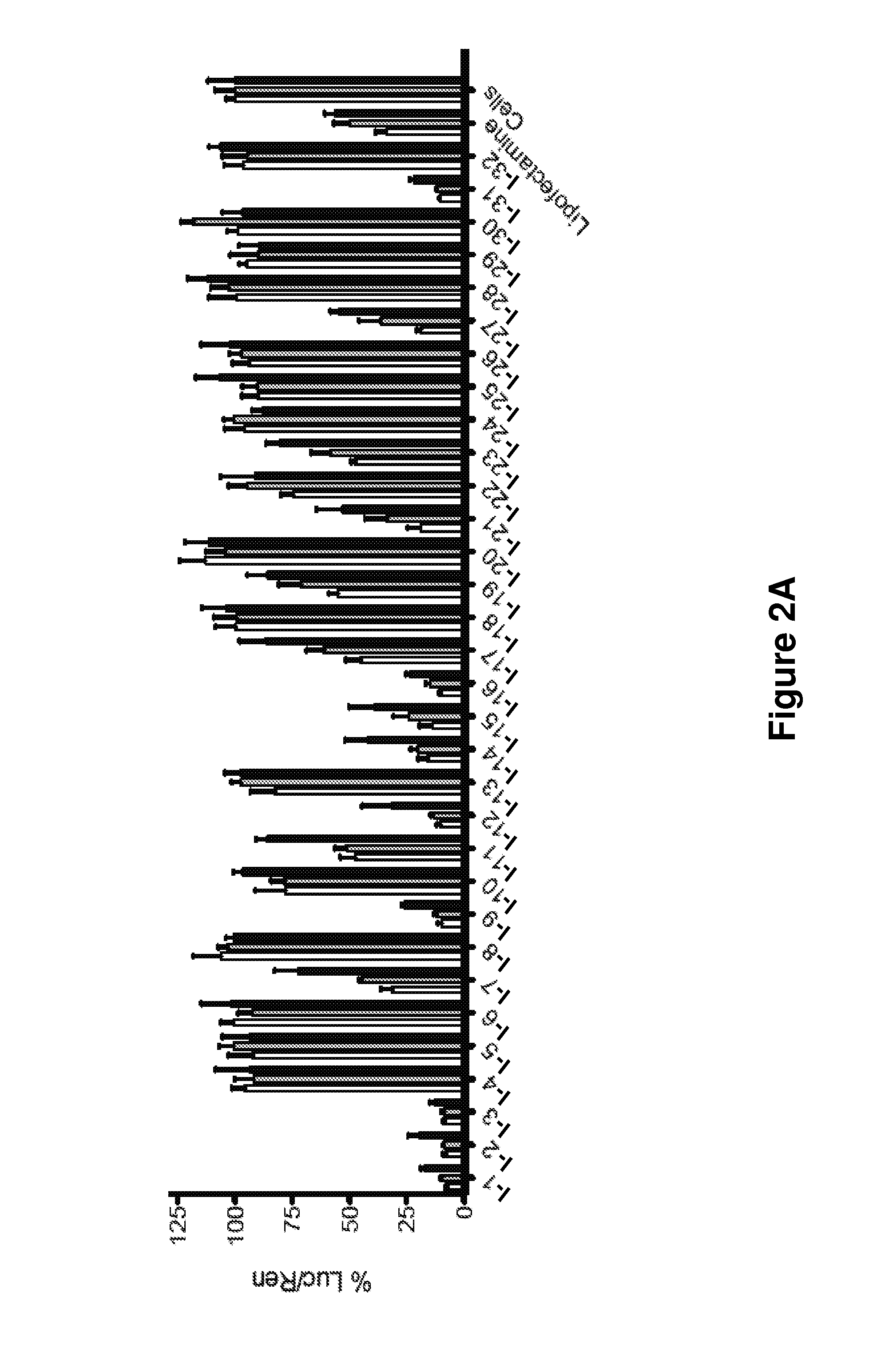
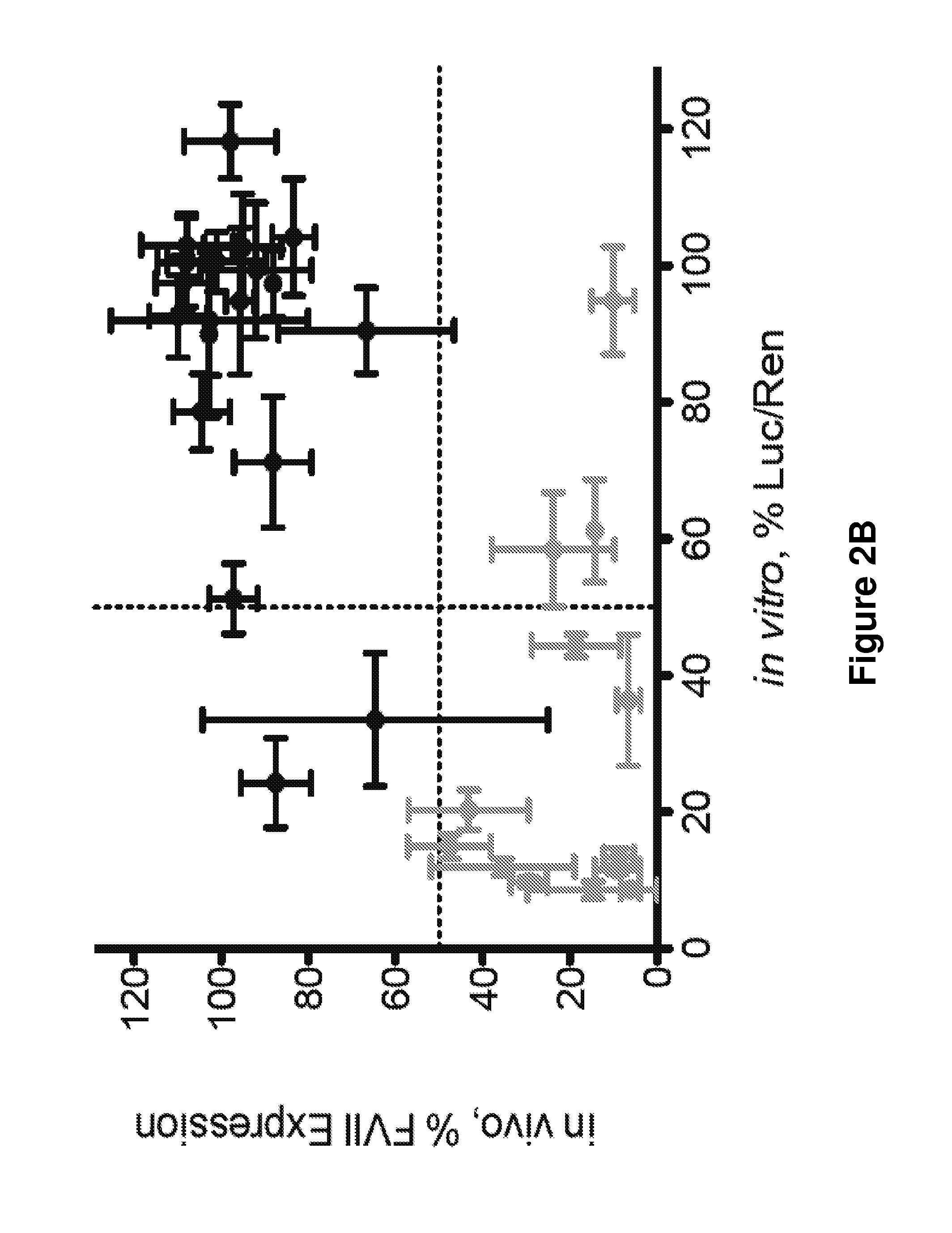
[0349]What is described herein is a systematic evaluation of multiple parameters associated with both the physicochemical properties and biological barriers to delivery for a group of lipid nanoparticles (LNPs). The approach involves mapping out the entire delivery pathway and evaluating the correlation between each property or delivery barrier and gene silencing (FIG. 1, steps 1-6). This systematic approach presents two potential advantages. First, the correlation of multiple physicochemical properties and biological barriers for a large set of LNPs with gene silencing allows for identification of relevant relationships between structure, biological function, and biological activity. Understanding these relationships will help improve the design of future therapeutic delivery vehicles. Second, a multi-parametric evaluation with LNPs may lead to the identification of parameters that can complement in vitro gene knockdown as a pre-screening tool for th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Stereoisomer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com