High capacity redox electrodes
a redox electrode, high-capacity technology, applied in the direction of diaphragms, sludge treatment, biomass after-treatment, etc., can solve the problems of film electrochemical inactivation, electrode destruction, conducting film degradation,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
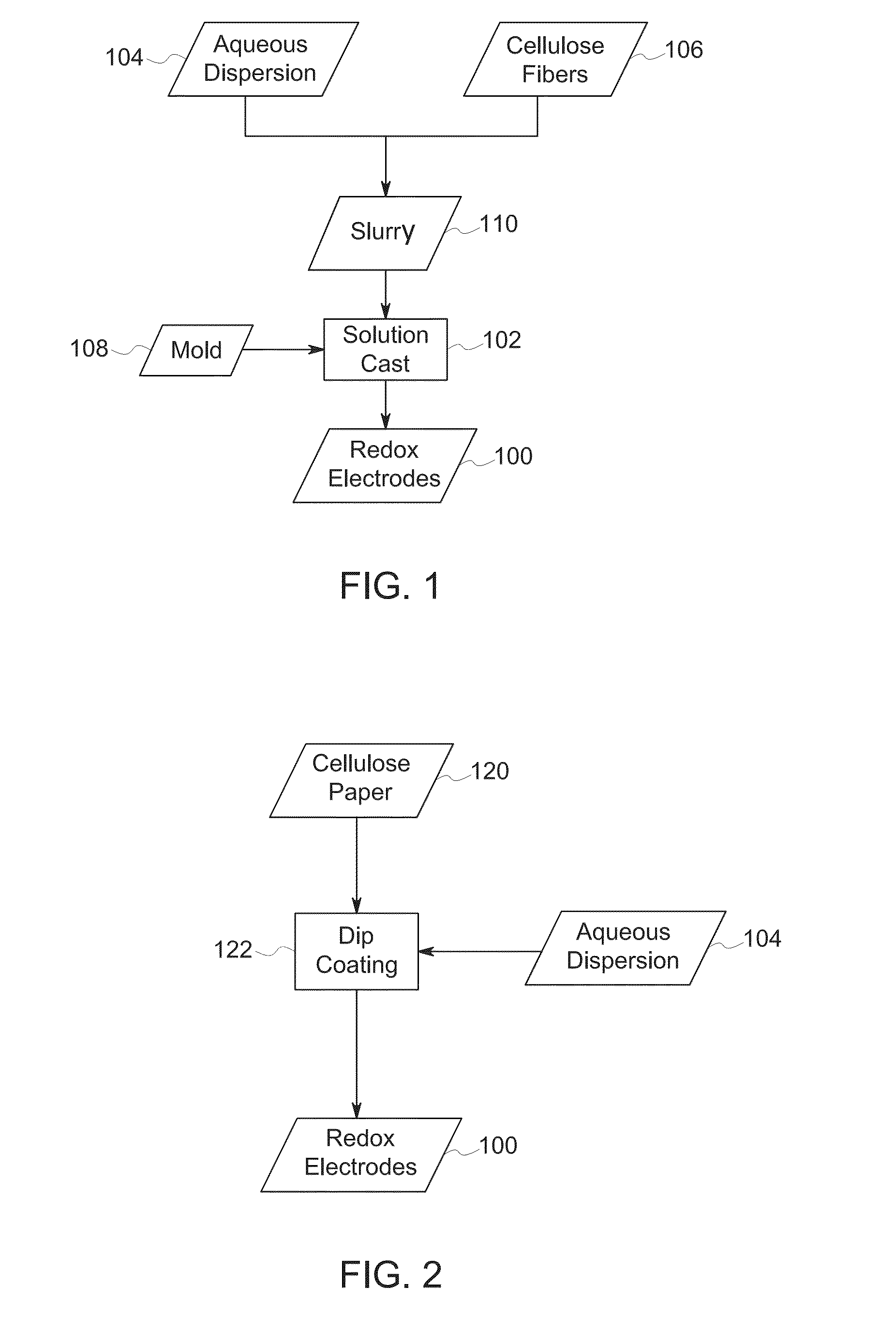
[0024]Turning to FIG. 1, in a first embodiment, redox electrodes 100 may be manufactured by solution casting (block 102) films of a PEDOT:PSS aqueous dispersion 104 blended with cellulose fibers 106. Various form factors of the electrodes 100 can be manufactured using this method (e.g., rods) by casting the solution into molds 108 of desired shape. As noted above, redox electrodes 100 formed in this manner may have a thickness greater than 1 micron relative to the underlying substrate surface.
[0025]By way of example, in this embodiment, an aqueous dispersion 104 of PEDOT:PSS (e.g., 1.0 wt %-1.3 wt %) blended with raw cellulose fibers 106 (10 μm to 5000 μm in length, which may be purchased in fiber form or generated through a ball milling process of cellulose paper). The PEDOT:PSS aqueous dispersion 104 is blended with the cellulose fibers to produce a slurry 110 that can be solution cast (block 102) into molds 108 to produce the electrodes 100 of different dimensions and form factor...
second embodiment
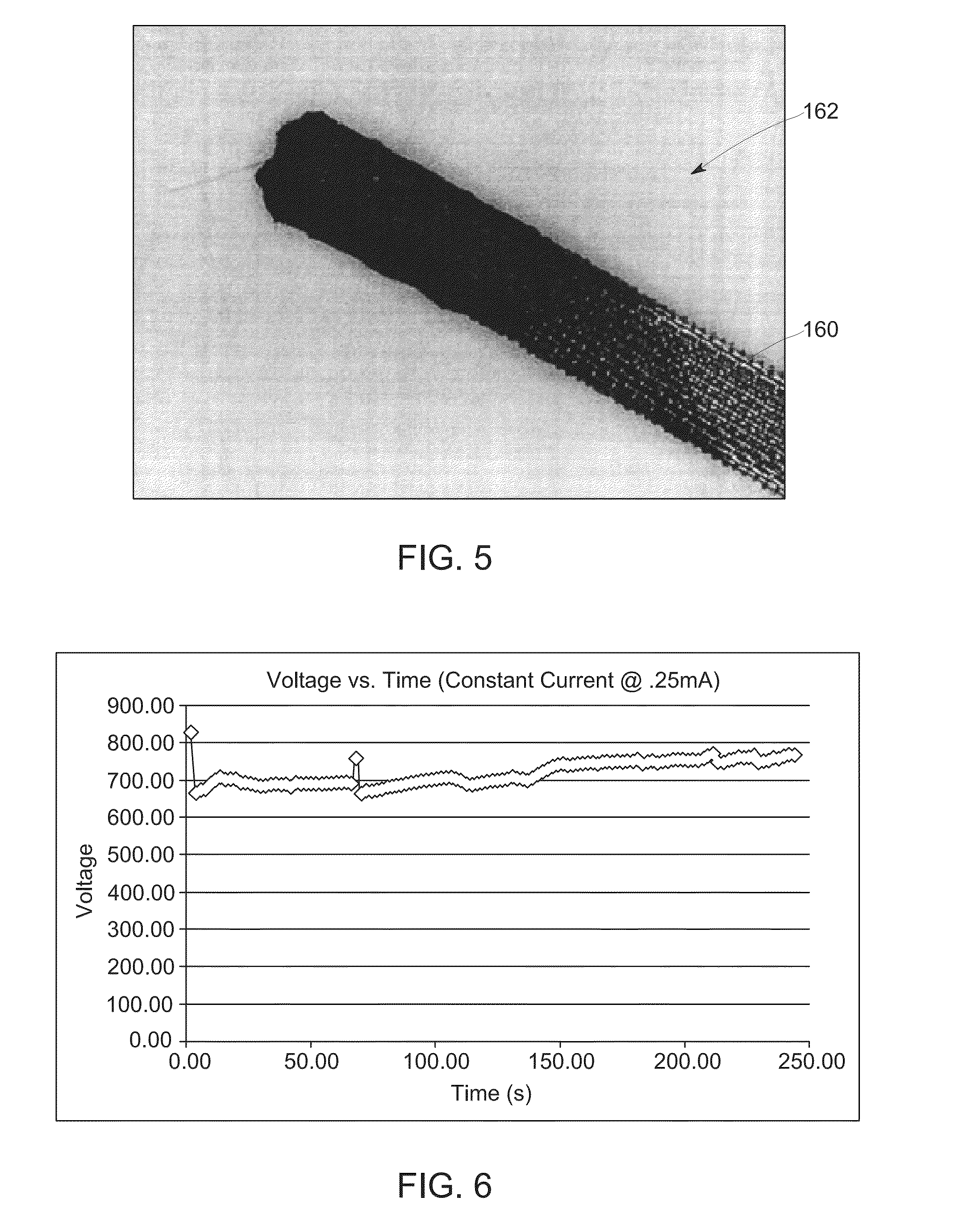
[0029]In a second embodiment, instead of solution casting, a dip coating approach may be employed. In one such embodiment, cellulose paper 120 cut to a desired dimension (e.g., Whatman 31 ETF paper) may be dip coated (block 122) into a PEDOT:PSS aqueous dispersion 104. In one such implementation, the cellulose paper may be dipped into the aqueous dispersion 104 for a length of time that yields the desired thickness of film forming the electrode 100 and yields the desired resistance values of the film. In other implementations, the cellulose paper 120 may be repeatedly dipped in the aqueous dispersion 104 to obtain the desired thickness and resistance values of the film that will form the redox electrode 100. By way of example, in one test, after four dip coat applications and drying processes, a uniform PEDOT:PSS coated cellulose paper was obtained suitable for use as an electrode 100 and having a resistance of less than 1 kΩ (at 4 mm probe distance).
third embodiment
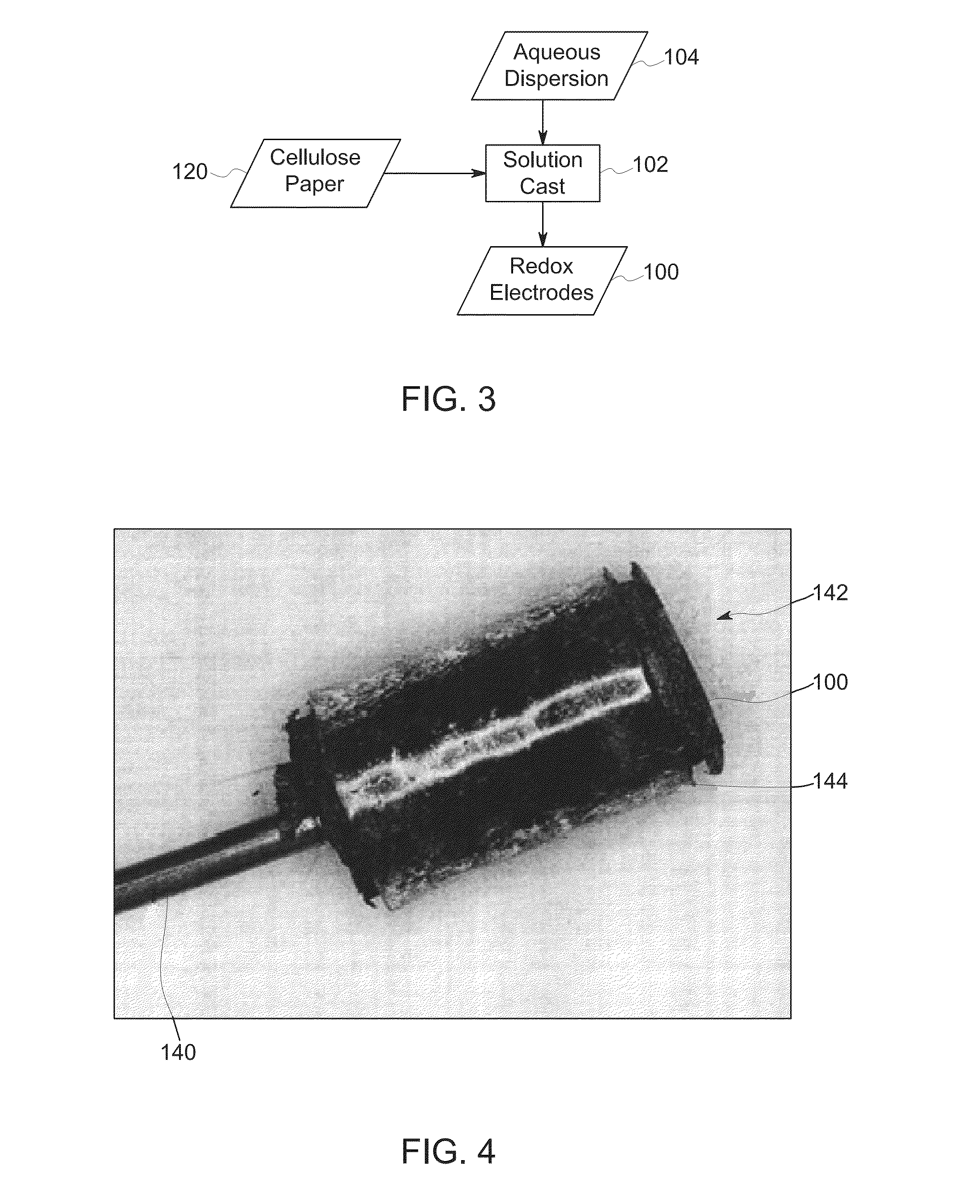
[0030]In a third embodiment, another solution casting approach may be employed. In this embodiment, a PEDOT:PSS aqueous dispersion 104 is solution cast (block 102) onto cellulose paper 120 (e.g., Whatman 31 ETF paper) until desired thickness of film and resistance values of film are obtained to form redox electrodes 100. In one implementation, the cellulose paper 120 is housed in an aluminum frame and heated at high temperature (e.g., 100° C.-150° C.). In this example, after six coats (e.g., three on each side), followed by drying and cross-linking, a uniform PEDOT:PSS coated cellulose paper was obtained suitable for use as a redox electrode 100 and having a resistance of less than 1 kΩ (at 4 mm probe distance).
[0031]With the preceding discussion of manufacturing approaches in mind, and turning to FIG. 4, in certain embodiments the surface area of the lead or wire 140 (e.g., copper wire) that is in contact with the PEDOT:PSS electrode may be increased. By way of example, a copper wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com