Methods for determining drug efficacy for the treatment of diffuse large b-cell lymphoma, multiple myeloma, and myeloid cancers
a technology for multiple myeloma and myeloid cancer, applied in the direction of instruments, transferases, peptide/protein ingredients, etc., can solve the problems of limited treatment options, limited leukemia in humans, and inability to treat cancer, so as to predict or monitor the responsiveness of a subject, monitor the compliance of a subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
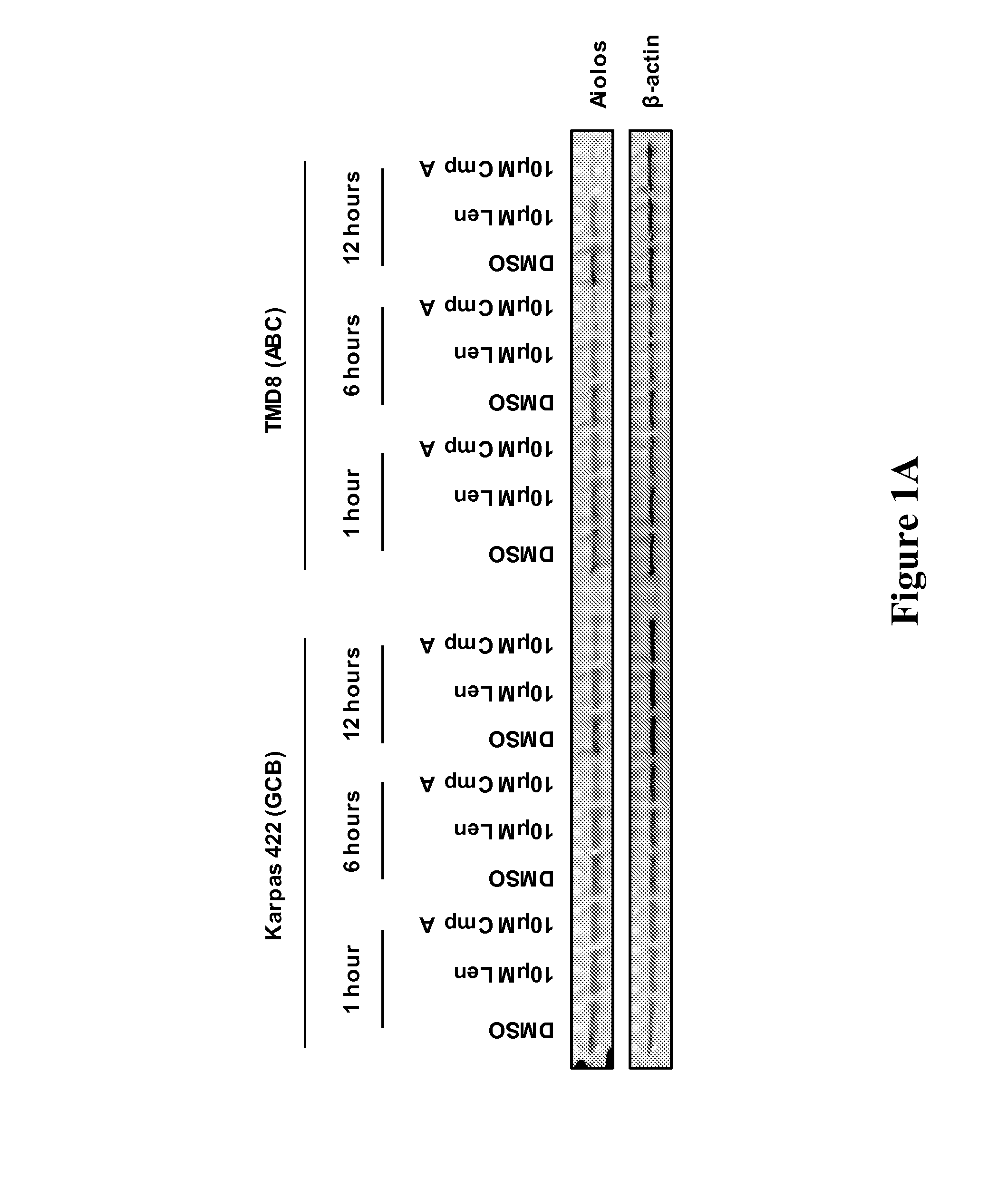
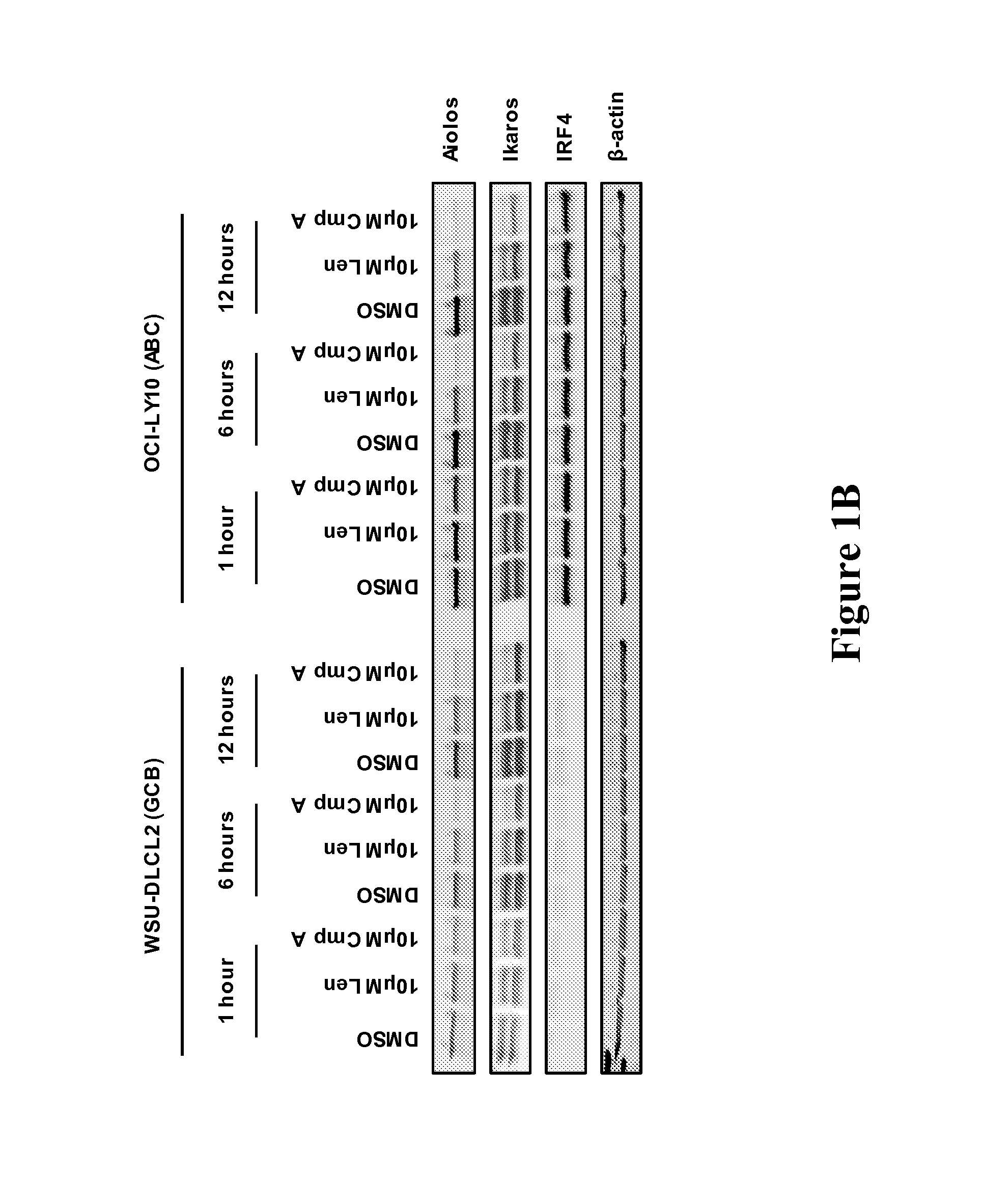
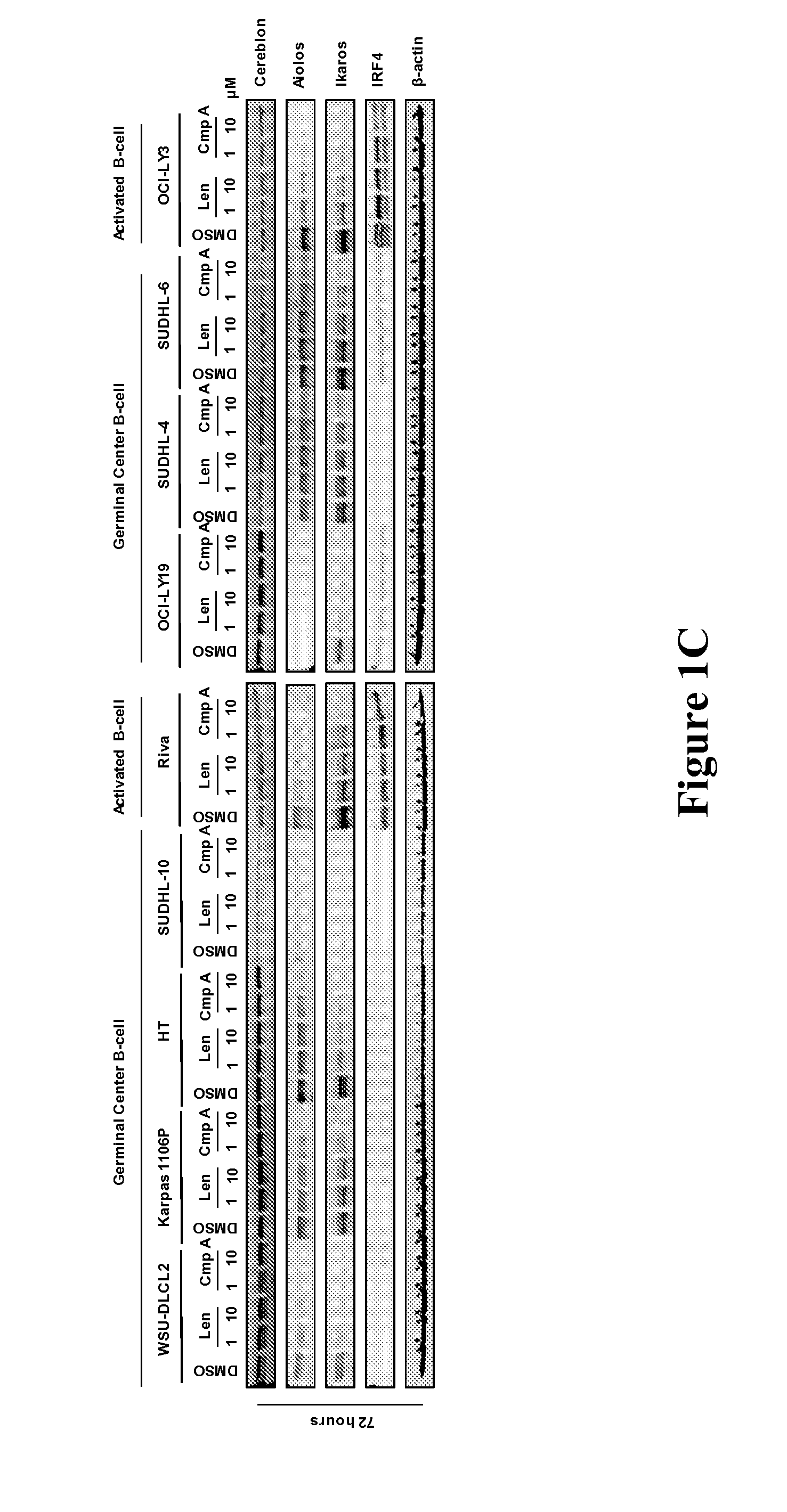
[0167]The methods, arrays, probes, and kits provided herein are based, in part, on the finding that a changed level, e.g., an increased level and / or a decreased level, of certain molecules (e.g., mRNAs, cDNAs, or proteins) in a biological sample can be utilized as biomarkers to predict responsiveness of a subject having or suspected to have a cancer (e.g., DLBCL, MM, MDS or AML) to a treatment compound (e.g., thalidomide, lenalidomide, pomalidomide, Compound A, or Compound B, or a stereoisomer thereof, or a pharmaceutically acceptable salt, solvate, hydrate, co-crystal, clathrate, or a polymorph thereof).
5.1 Definitions
[0168]As used herein, and unless otherwise specified, the terms “treat,”“treating” and “treatment” refer to an action that occurs while a patient is suffering from the specified cancer, which reduces the severity of the cancer, or retards or slows the progression of the cancer.
[0169]The term “sensitivity” and “sensitive” when made in reference to treatment with compou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com