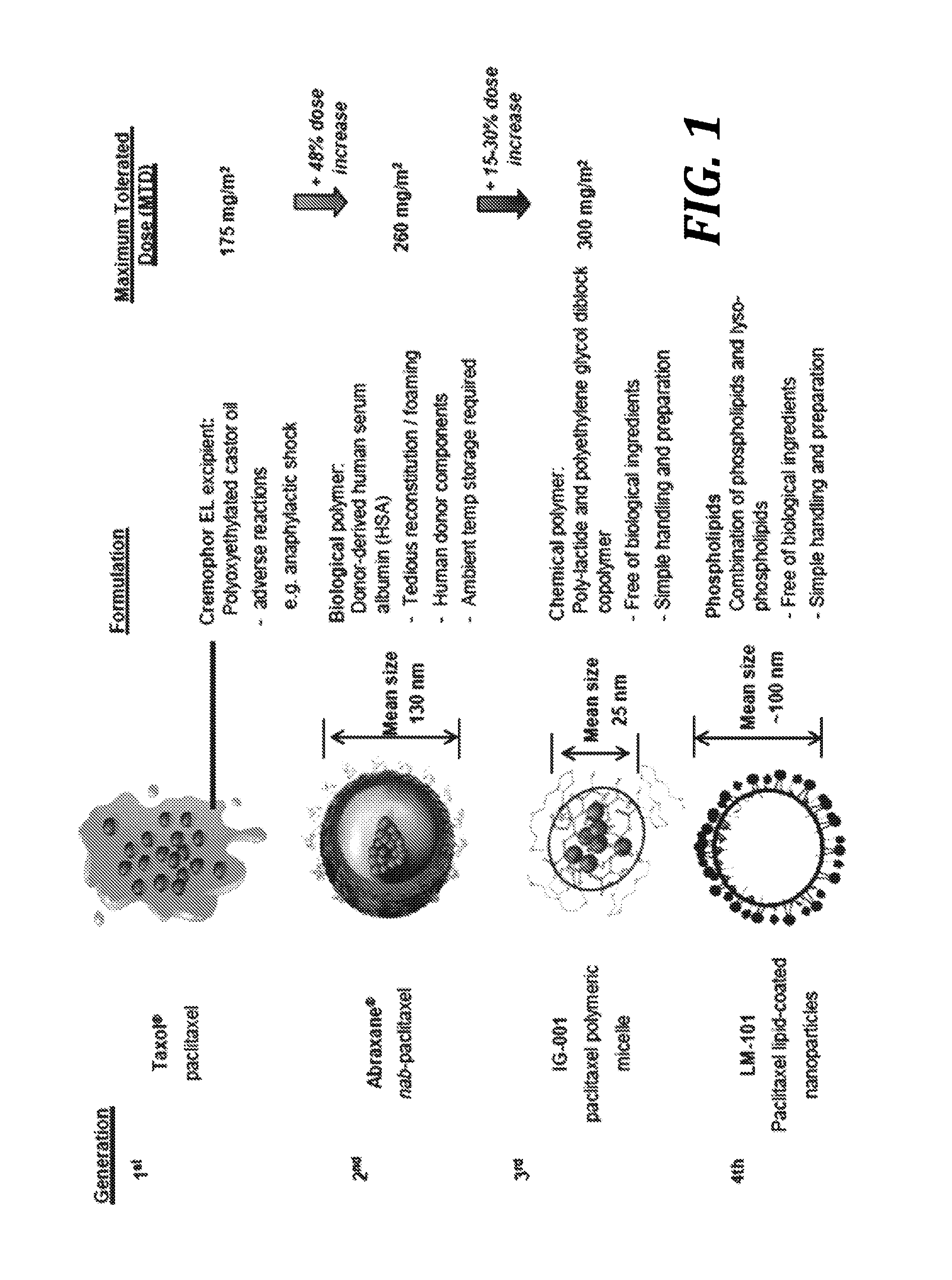
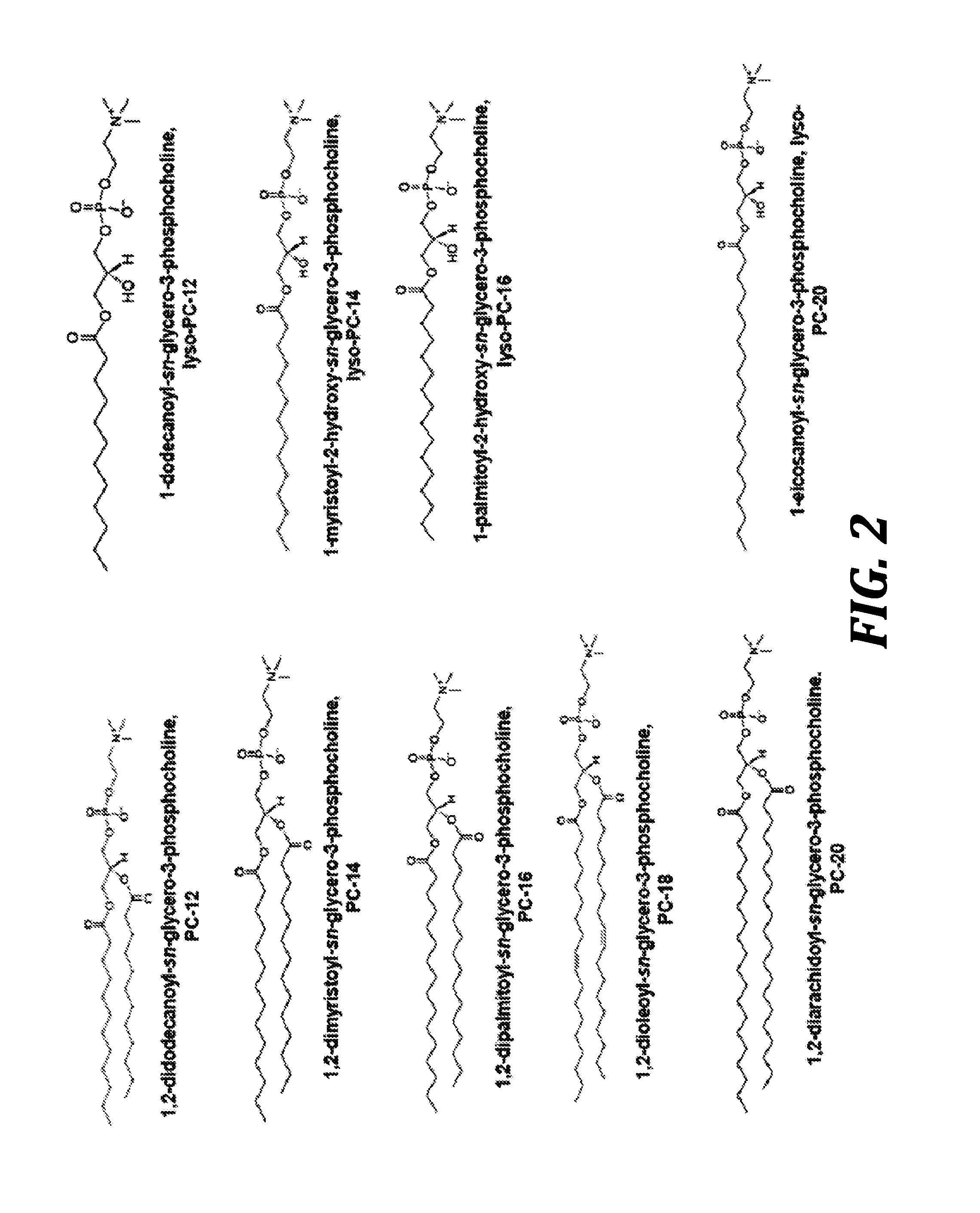
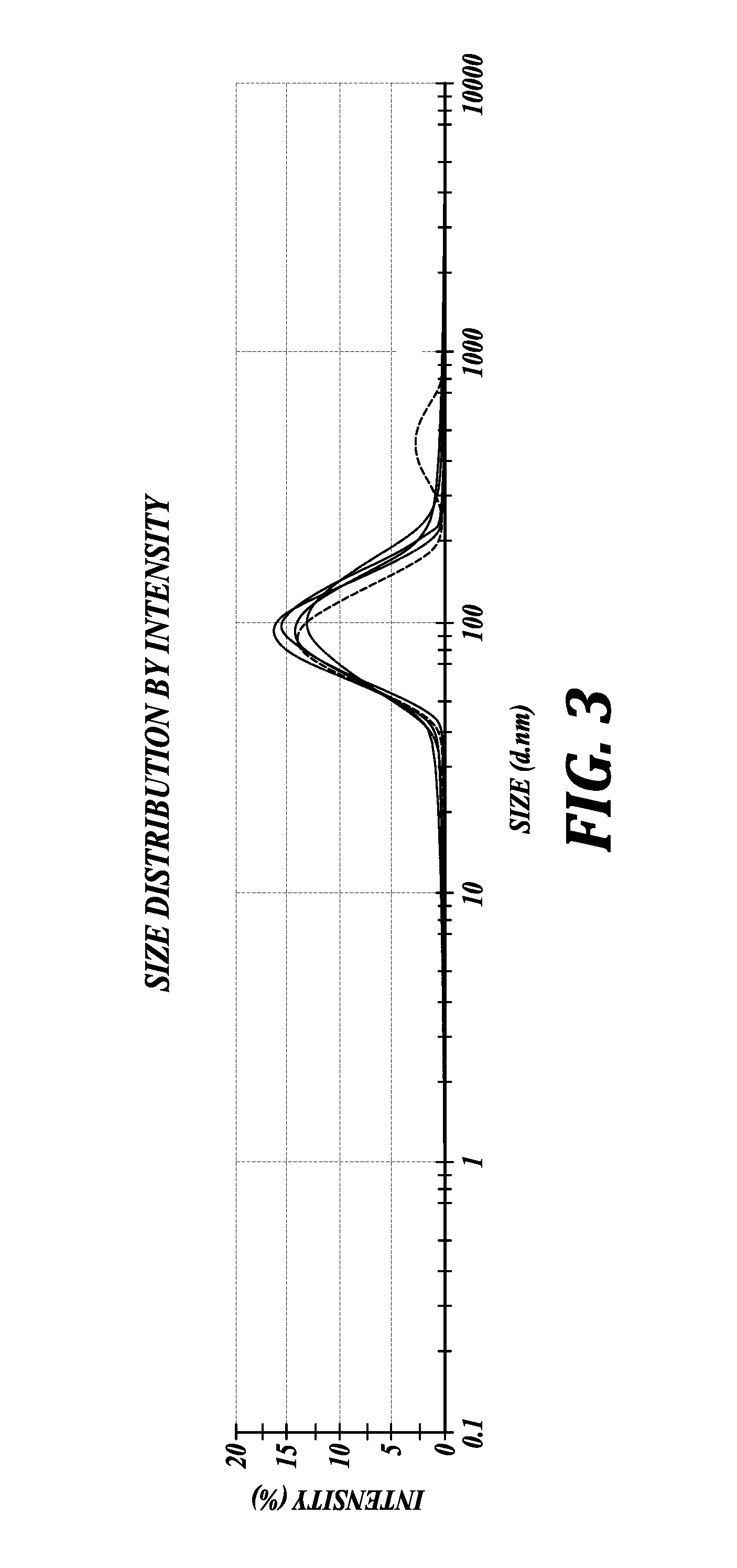
Phospholipid-coated therapeutic agent nanoparticles and related methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Preparation of Representative Paclitaxel Nanoparticles by Microfluidization-Solvent Evaporation
[0231]The PTX-phospholipid NPs were prepared by LV1 low volume Microfluidizer® processor (Microfluidics, Massachusetts, US) microfluidization. The organic solvent (ethanol:chloroform (9:1)) containing PTX and phospholipids were added to an aqueous phase (de-ionized water, DI) and the emulsion was run through the microfluidizer to provide a nanoemulsion. The solvent from the nanoemulsion was removed by rotary evaporation to provide a nanosuspension of phospholipid-coated PTX nanoparticles in the aqueous phase.
[0232]Nanoparticles were prepared by mixing together the organic and aqueous phase. Organic phase consisted of 40 mg of 12:0 PC (DLPC) 1,2-dilauroyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Inc., Alabama, US), 10 mg of 12:0 lyso PC 1-lauroyl-2-hydroxy-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Inc., Alabama, US) and 10 mg of paclitaxel (paclitaxel was from Tecoland ...
example 2
The Preparation of Representative Paclitaxel Nanoparticles by Thin-Film Hydration
[0234]A phospholipid film was prepared by dissolving PTX and phospholipids in ethanol. The dry film was hydrated with water for visual, microscopic, size and loading efficiency measurements of the resulting unfiltered formulation.
[0235]Organic phase consisted of 40 mg of 12:0 PC (DLPC) 1,2-dilauroyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Inc., Alabama, US), 10 mg of 12:0 lyso PC 1-lauroyl-2-hydroxy-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Inc., Alabama, US) and 10 mg of paclitaxel (paclitaxel was from Tecoland Corporation, Irvine, Calif. (DMF No. 11909)) dissolved in 10 ml of ethanol. Aqueous phase consists of 10 ml DI water. The two phases are mixed in a 250 ml evaporator flask. The solution is completely evaporated using a rotary evaporator at the following conditions: water bath temperature=28° C., pressure <2 mm Hg, chiller temperature=5° C., rotation speed=280 rpm, until a film ...
example 3
Size and Zeta Potential Measurement of Representative Paclitaxel Nanoparticles
[0236]The particle size and the particle size measurements were carried out using Zetasizer Nano-ZS (Malvern Instruments Ltd, Worcestershire, UK) and the Zav hydrodynamic diameter of the samples was determined by cumulative analysis. The particle size and particle size distribution by intensity were measured by photon correlation spectroscopy (PCS) using dynamic laser light scattering (4 mW He—Ne laser with a fixed wavelength of 633 nm, 173° backscatter at 25° C.) in 10 mm diameter cells. The Zav of the particle size, also known as cumulants mean, is defined as harmonic intensity average particle diameter. All measurements were done with six runs. Zeta potential (surface charge) determinations of the NPs in water were based on the electrophoretic mobility of the particles using folded capillary cells in automatic mode of measurement duration using Zetasizer Nano-ZS. The measurements were performed by the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap