Salts and polymorphs of cyclic boronic acid ester derivatives and therapeutic uses thereof
a technology ester derivatives, which is applied in the field of salts and polymorphs of cyclic boronic acid ester derivatives, can solve the problems of threatening the clinical utility of antibacterial therapy, higher morbidity and mortality, and long patient hospitalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Salt Screening Study
Step 1. Purification and Characterization of Compound 1
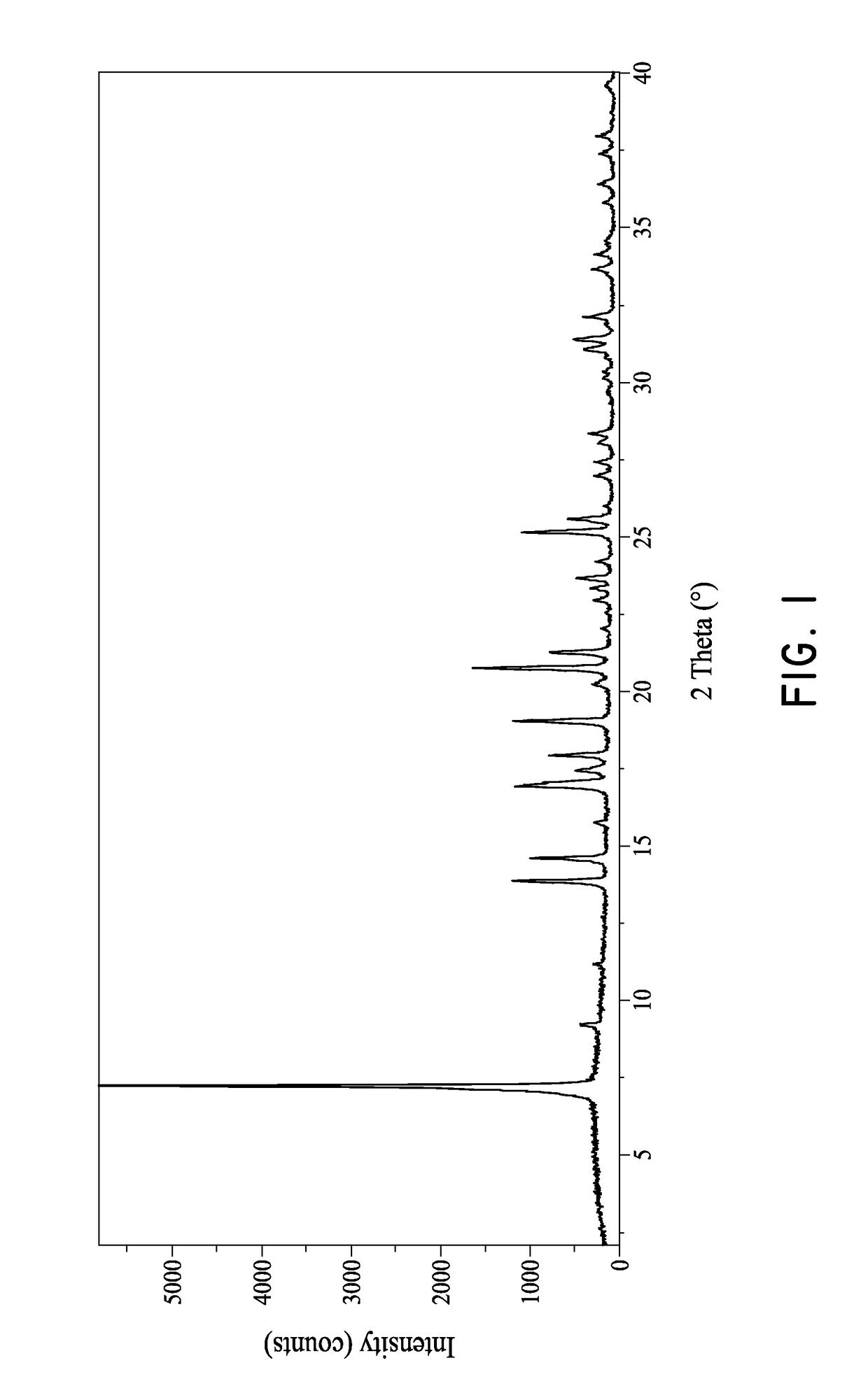
[0083]Compound 1, 2-((3R, 6 S)-2-hydroxy-3-(2-(thiophen-2-yl)acetamido)-1,2-oxaborinan-6-yl)acetic acid, was found to often contain undesired NaCl salt. FIG. 6 shows the PXRD pattern of the NaCl, Compound 1 before purification and after purification. As shown in FIG. 6, the peak at 31.6° 2θ in the PXRD pattern is characteristic for the NaCl salt. The PXRD pattern for Compound 1 after purification shows significantly reduced or no presence of the NaCl salt.
[0084]During the purification process, compound 1 was combined with water (15.0 mL) and the suspension was stirred at room temperature for 4 hrs. The solids were isolated using a Buchner funnel and Whitman #1 filter paper. After filtration, the compound was air-dried for 15 hrs. The yield of the procedure was 87% by weight.
[0085]After the purification step, the purified compound was analyzed using HPLC, PXRD and TGA. The PXRD pattern of the purified compound...
example 2
Preparation of Potassium Salt at 50 mg Scale
[0098]48.7 mg of Compound 1 was combined with acetone (1.0 mL) in a 2-mL vial containing a stir bar. The suspension was stirred at 40° C. for 10 min and seeded with about 1 mg of potassium salt. 164.0 μL of potassium t-butoxide (1.0eq. of compound 1; 1.0M solution in t-BuOH) was added in five aliquots: 10 μL, 20 μL, 20 μL, 20 μL, 93 μL in every 5 min. Gum was formed upon the counterion addition. The sample was stirred for 15 hrs while the cycling temperature was maintained between 40° C.-45° C., during which the gum changed into a free-flowing suspension. The suspension was equilibrated at 5° C. for 30 min and the solids were isolated on a Buchner funnel under nitrogen blanket and allowed to dry for 30 min. The yield of this preparation procedure was 70% wt.
example 3
Preparation of Potassium Salt at 300 mg Scale
[0099]Compound 1 (302.6 mg) was combined with ACN (15.0 mL) in a 20-mL vial containing a stir bar. The suspension was stirred at 50° C. for 30 min and seeded with about 3 mg of potassium salt. 256.7 μL of potassium hydroxide (0.9 eq. of compound 1; 3.57M aqueous solution) was added in eight aliquots: 20 μL, 20 μL, 20 μL, 20 μL, 20 μL, 50 μL, 50 μL, 56.7 μL in every 15 min. Partial conversion to a gum upon the counterion addition was observed. The suspension was stirred at 50° C. for 60 min, cooled to 5° C. at 0.1° C. / min rate (7.5 hrs), and subjected to stirring while the cycling temperature was maintained between 40° C.-45° C. for 10 hrs. The temperature cycle involved holding at 40° C. for 1 hr followed by cooling at 0.5° C. / min, and then holding at 5° C. for 2 hrs followed by heating to 40° C. at a maximum rate. The suspension was equilibrated at 5° C. for 2 hrs. The solids were isolated on a Büchner funnel under nitrogen blanket, allo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| 2θ | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com