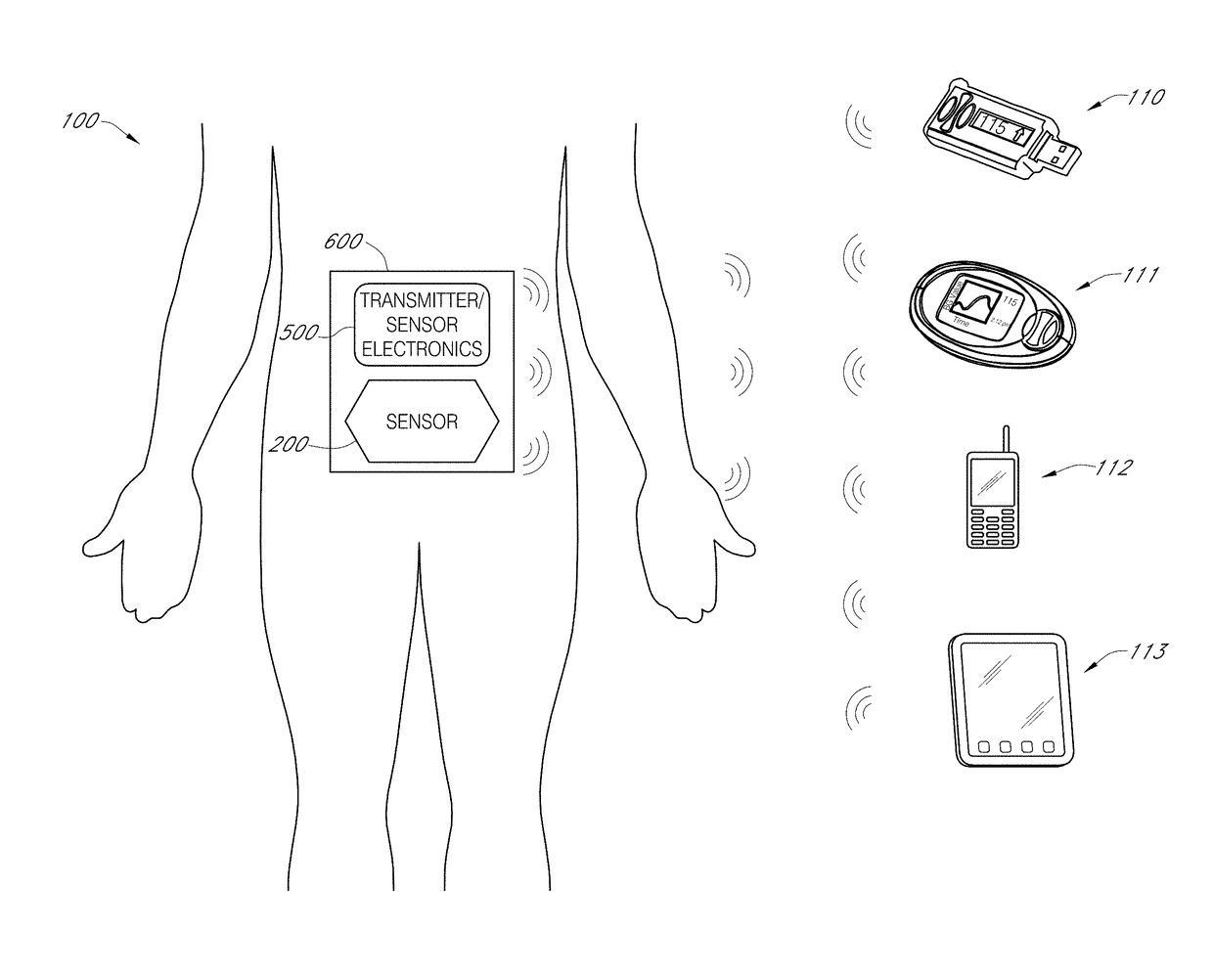
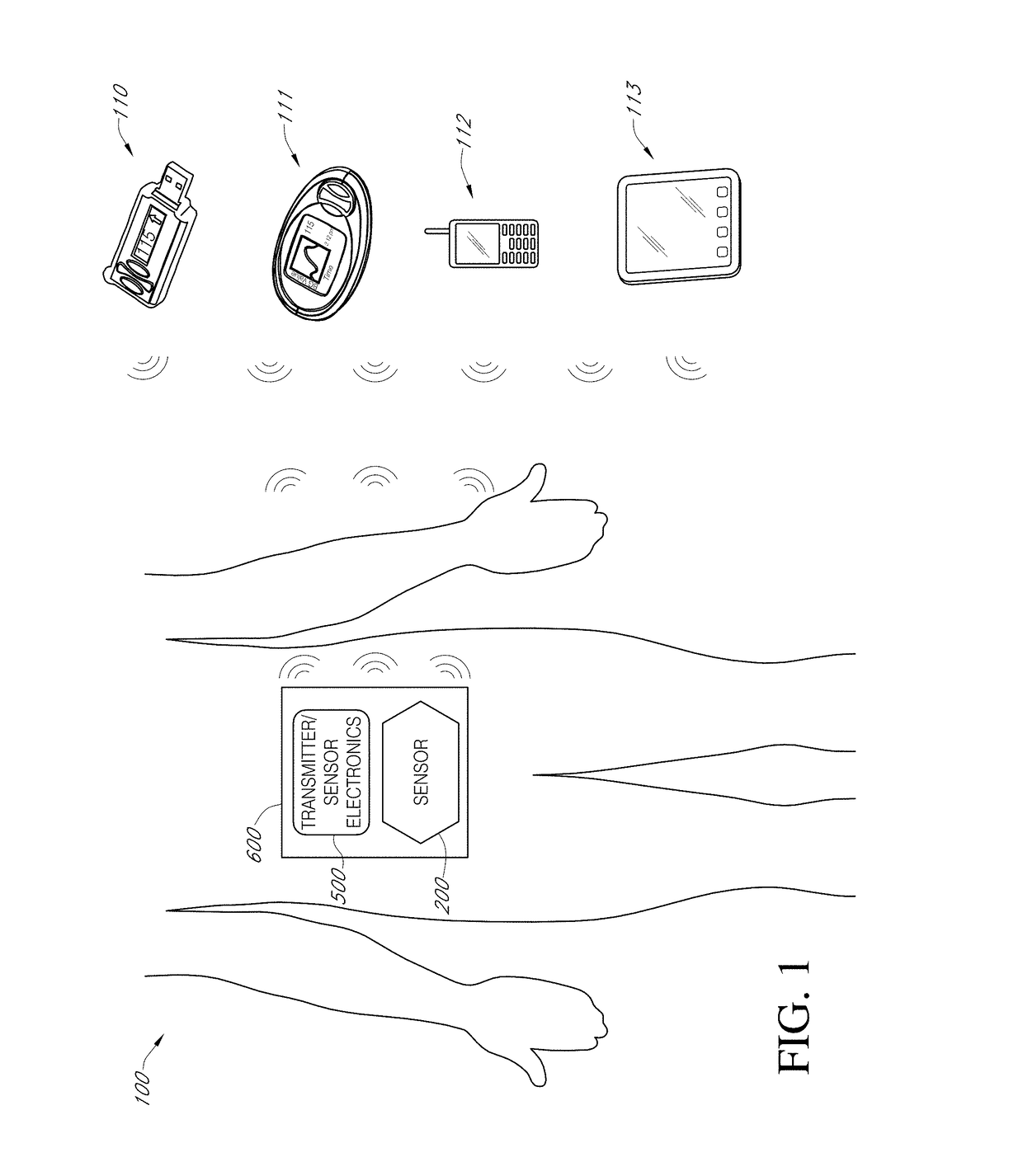
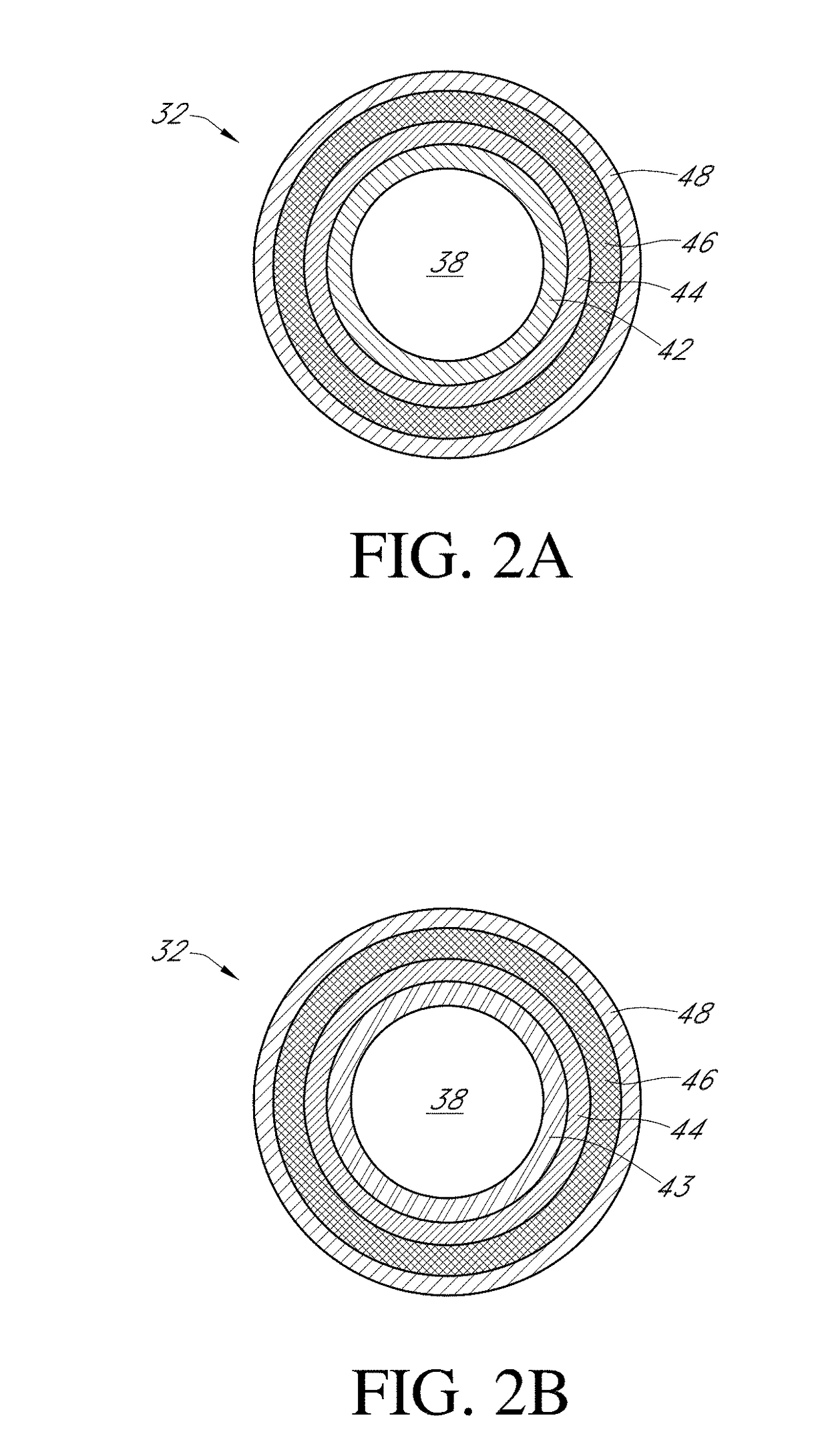
Biointerface layer for analyte sensors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Biointerface Polymers and Characterization
[0317]Various biointerface polymers were prepared using different amounts of hard segments, PEG, and sulfobetaines. The hard segments (HS) were polyurethanes or polyureas prepared from diisocyanates reacted with diol or diamine chain extenders of less than 12 carbon units. The particular formulations are shown in Table 1.
TABLE 1PEGBetaineHSMnPDIName(wt. %)(wt. %)(wt. %)(Da)(Mw / Mn)SBL-3201835107,0001.7SBL-10354025127,0001.6SBL-12103561,0001.6SBL-927343177,4001.7SBL-8232735140,0002.1RL-7*004245,0001.7*Comparative.
example 2
Characterization Analysis
[0318]Sensors were built as described in the section entitled “sensor systems.” The in vitro response time was tested and compared to a sensor without the biointerface layer. It was found that the sensor with the biointerface layer had the same T95 response time as the sensor without the biointerface layer, indicating that the biointerface layer did not slow the response times of a glucose sensor (FIG. 5)
[0319]The in vivo response time was also tested in pig over the course of 15 days. In particular continuous sensors with SBL-3 and crosslinked SBL-9 biointerface layers were compared to a sensor without a biointerface layers. It was likewise found that there was no difference in response times for the sensors with and without the biointerface layer (FIG. 6). This again showed that the biointerface domain did not affect the glucose sensor response time.
[0320]The cal-check performance of sensors without a biointerface layer and sensors dip coated with 5 wt. % ...
example 3
Antifouling Properties
[0333]The mechanisms of protein adsorption and antifouling properties of the biointerface layer were explored. The use of zwitterions embedded within and physically, rather than covalently, contained in a polymer are believed to work via the migration of these hydrophilic species to the interface between the polymer and the environment. Biointerface layer made from SBL-10 was tested by X-ray photoelectron spectroscopy (XPS) in a dry state and after soaking. It was found that the atomic concentration of Sulfur on the SBL-10 coated surface of sensor tip did not increase before soaking (dry state, 0.3%) and after soaking (0.2%), which indicate there was no migration to the surface of the zwitterionic segments in the polymer chain (FIG. 13). Thus, while not wishing to be bound by theory, it is believed that the biointerface layer creates a loosely bonded water layer at the surface which prevents the adsorption of proteins and cells (FIG. 12).
[0334]As another theory...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Atomic weight | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
| Contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com