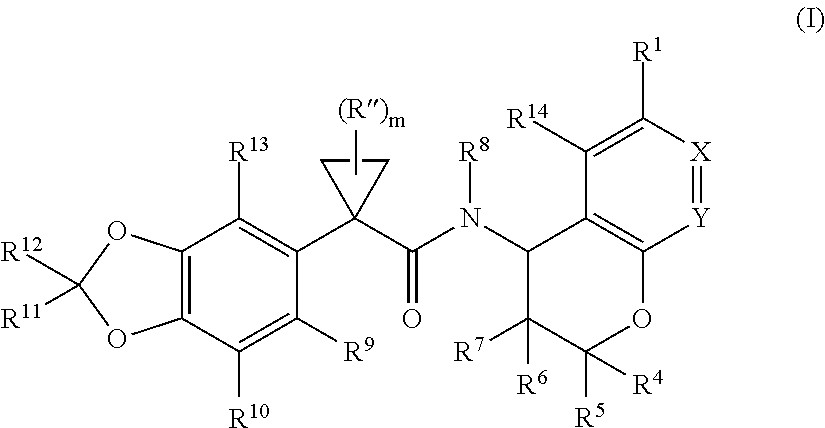
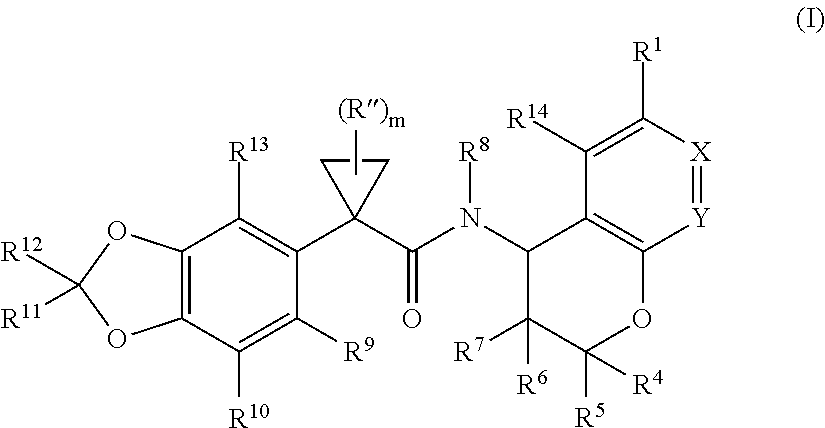
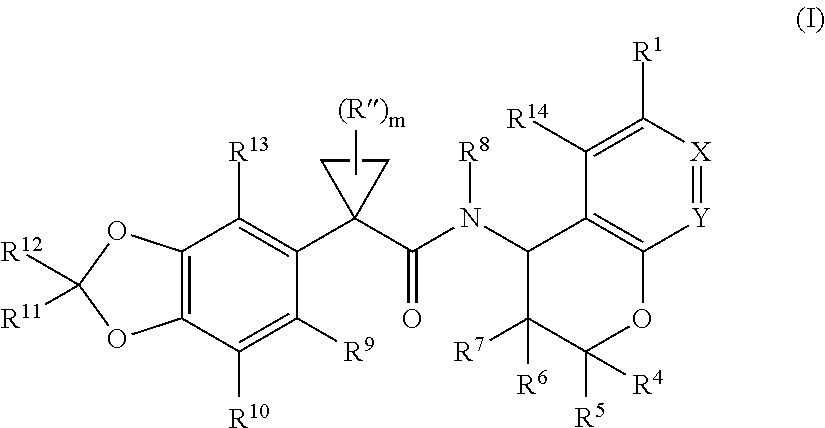
Substituted chromanes and method of use
a technology of substituted chromanes and chromane compounds, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, organic chemistry, etc., can solve the problems of premature death of affected patients, irreversible lung damage, and mucus dehydration, and achieve low drug-drug interaction potential, low effect on cyp3a4 expression, and improved potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-[(2R,4R)-4-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-7-methoxy-3,4-dihydro-2H-chromen-2-yl]benzoic acid
[1738]To a solution of Example 6 (25 mg, 0.047 mmol) in tetrahydrofuran (233 μL) was added lithium hydroxide hydrate (233 μL of a 0.8 M solution in water). The resulting biphasic mixture was stirred vigorously for 16 hours at room temperature, followed by addition of more lithium hydroxide hydrate (233 μL of a 0.8 M solution). The reaction mixture was stirred for an additional 5 hours at room temperature, acidified by the addition of 6 M HCl (0.040 mL) and the resulting biphasic mixture loaded directly onto a 4 g silica gel cartridge and eluted with 30% ethyl acetate / heptanes over 15 minutes to give the title compound as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 8.06 (dd, J=7.9, 1.5 Hz, 1H), 7.71-7.61 (m, 1H), 7.48 (t, J=7.7 Hz, 1H), 7.13 (dd, J=8.2, 1.7 Hz, 1H), 7.09 (d, J=1.7 Hz, 1H), 7.01 (d, J=8.2 Hz, 1H), 6.96 (d, J=8.6 Hz, 1H), 6.52 (d...
example 2
3-[(2R,4S)-4-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-7-methoxy-3,4-dihydro-2H-chromen-2-yl]benzoic acid
[1739]To a solution of Example 5E (35 mg, 0.065 mmol) in tetrahydrofuran (326 μL) was added lithium hydroxide hydrate (326 μL of a 0.8 M solution). The resulting biphasic mixture was stirred vigorously for 16 hours at room temperature, followed by addition of more lithium hydroxide hydrate (326 μL of a 0.8 M solution). The reaction was stirred for an additional 5 hours at room temperature, acidified by the addition of 6 M HCl (0.050 mL) and the resulting biphasic mixture was loaded directly onto a 4 g silica gel cartridge and eluted with 30% ethyl acetate / heptanes over 15 minutes to give the title compound as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.13 (t, J=1.8 Hz, 1H), 8.12-8.04 (m, 1H), 7.72-7.61 (m, 1H), 7.51 (t, J=7.7 Hz, 1H), 7.20-7.11 (m, 2H), 7.04 (dd, J=8.4, 4.1 Hz, 2H), 6.53 (dd, J=8.5, 2.6 Hz, 1H), 6.45 (d, J=2.6 Hz, 1H), 5.60 (d, J=6.6 Hz, ...
example 3
1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-[(2R,4R)-2-(3,4-dimethoxyphenyl)-7-methoxy-3,4-dihydro-2H-chromen-4-yl]cyclopropanecarboxamide
[1740]To 1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxylic acid (CAS 68015-98-5) (120 mg, 0.496 mmol) in DMF (1239 μL) was added HATU (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate) (245 mg, 0.644 mmol). The mixture was stirred for 5 minutes at room temperature, and then 2-(3,4-dimethoxyphenyl)-7-methoxychroman-4-amine (156 mg, 0.496 mmol) was added, followed by dropwise addition of triethylamine (276 μL, 1.982 mmol). After 45 minutes, the mixture was quenched with saturated aqueous sodium bicarbonate, and the aqueous layer removed. The resulting oil was triturated with water and filtered to give 283 mg of a white solid. The solid was dissolved in dichloromethane and purified using a 24 g silica gel cartridge with a gradient of 5-50% ethyl acetate / heptanes to give 189 mg of a mixture of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com