HERIPENES WITH PAIN-RELIEVING EFFECT, ACTIVE SUBSTANCES OF Hericium erinaceus MYCELIUM AND THE PREPARATION METHOD THEREOF, AND PHARMACEUTICAL COMPOSITION CONTAINING THE HERIPENES OR ACTIVE SUBSTANCES
a technology of hericium erinaceus and active substances, which is applied in the field of active substances with pain-relieving effects, can solve the problems of few effective drugs that thoroughly inhibit pain, difficult treatment, tolerance and dependence problems,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 3
ion of the Alcohol Extract Mixture
[0092]The freeze-dried powder and an equal amount of the alcohol extract were mixed, and then centrifuged to obtain the supernatant. The supernatant was freeze-dried to obtain a mixture of the H. erinaceus mycelia and the alcohol extract (hereinafter abbreviated as “the alcohol extraction mixture”).
embodiment 4
ion and Analysis of the Erinacine S Standard
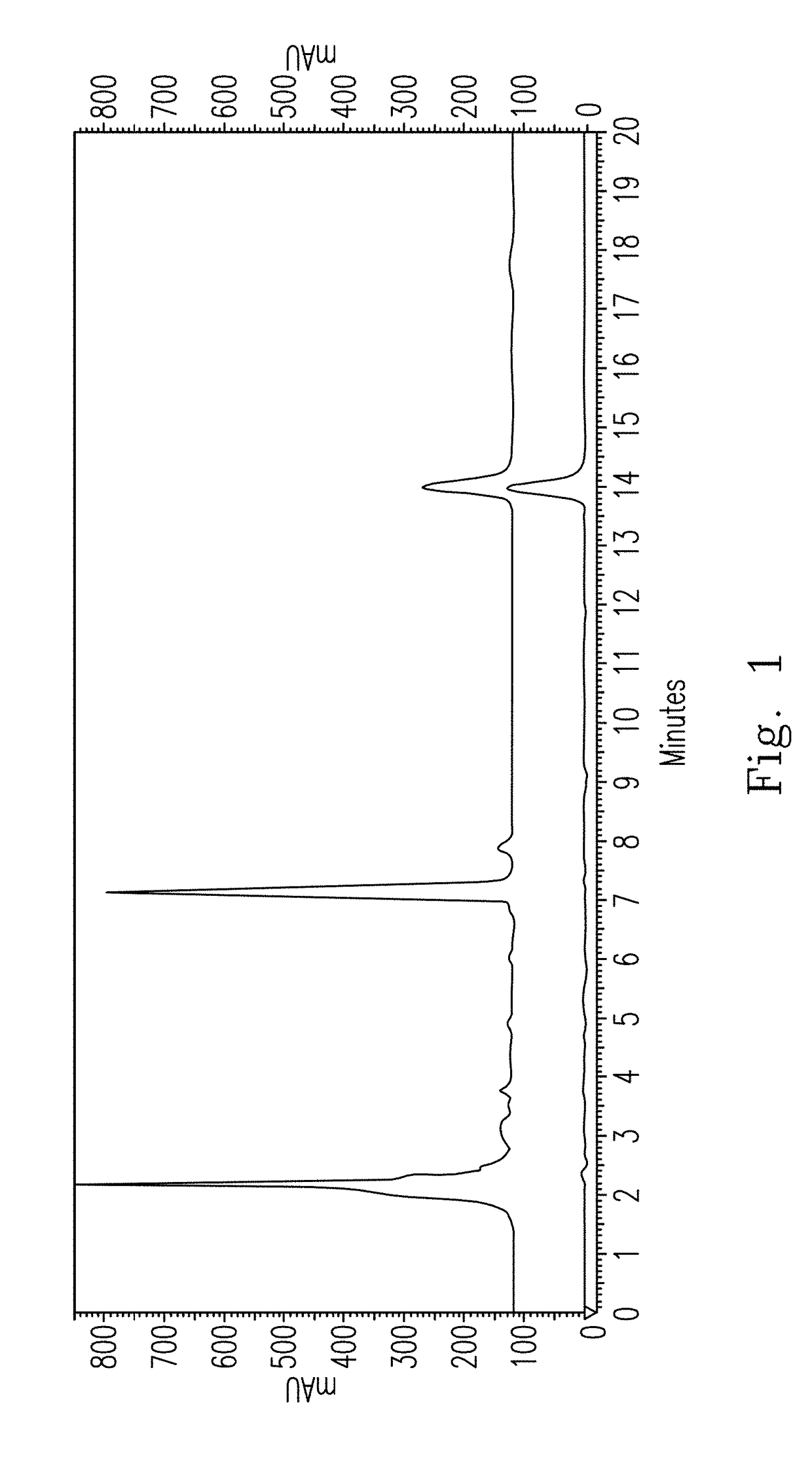
[0093]The preparation method for the erinacine S standard is described as follows. The H. erinaceus mycelia alcohol extract was subjected to liquid-liquid partition (H2O:ethyl acetate (EA)=1:4 (v / v)), and the obtained ethyl acetate extract was further subjected to column chromatography on silica gel and Sephadex® LH-20 silica gel, followed by the gradient elution of n-hexane:EA (10:1, 3:1, 3:2, 1:1, 1:2, 0:1; v / v), to obtain 7 sub-partitions. The sub-partition 3 (which was eluted with n-hexane:EA=3:2 (v / v)) was further subjected to column chromatography on the Sephadex LH-20 silica gel to obtain a new compound, erinacine S (i.e. formula (I)), whose structural formula was identified via chemical analysis, and characteristic analysis was performed via high performance liquid chromatography (HPLC). The erinacine S was obtained using a reverse chromatography column Cosmosil 5C18-AR-II (Nacalai USA, California, U.S.A.) at 40° C. and eluted usin...
embodiment 5
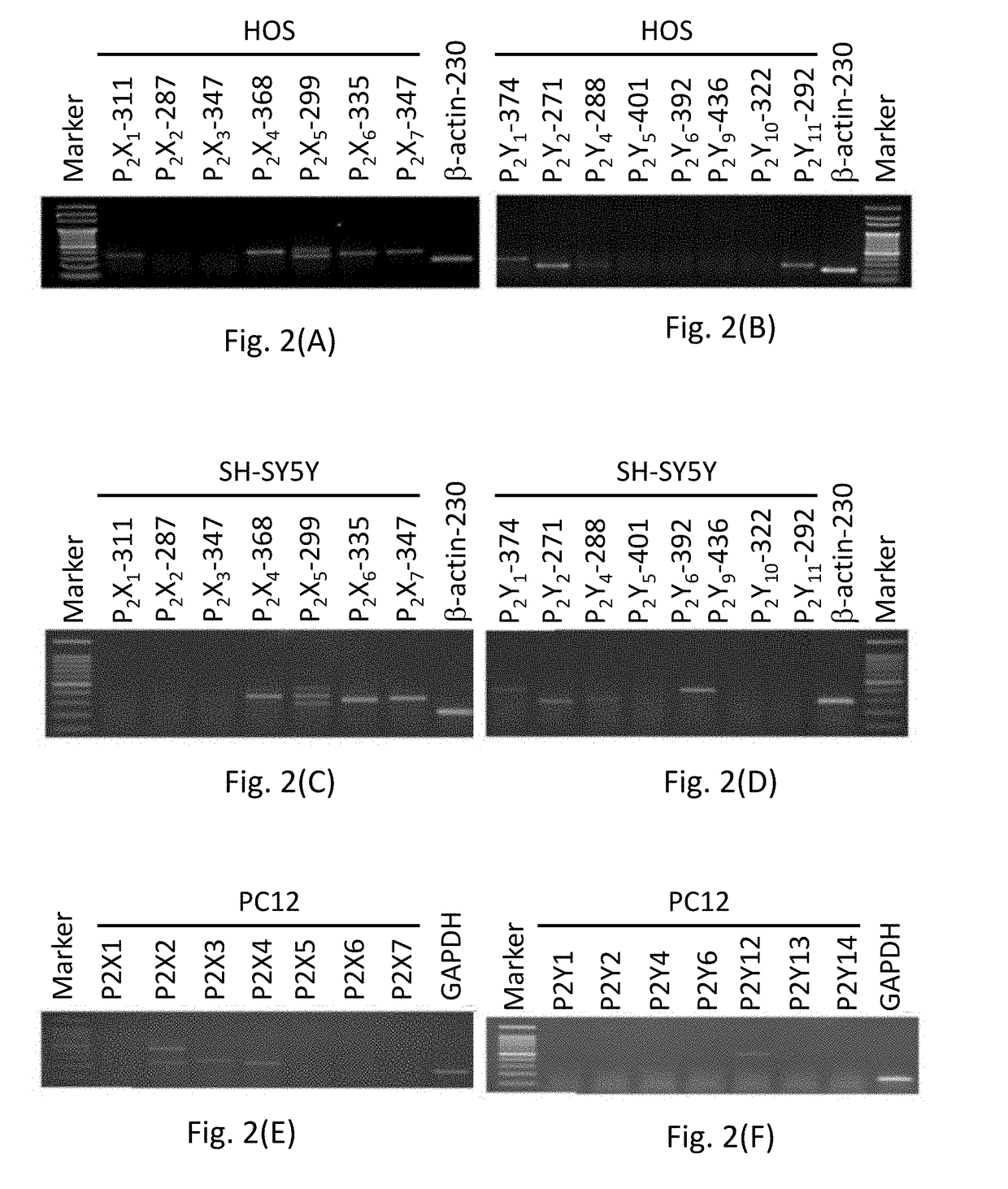
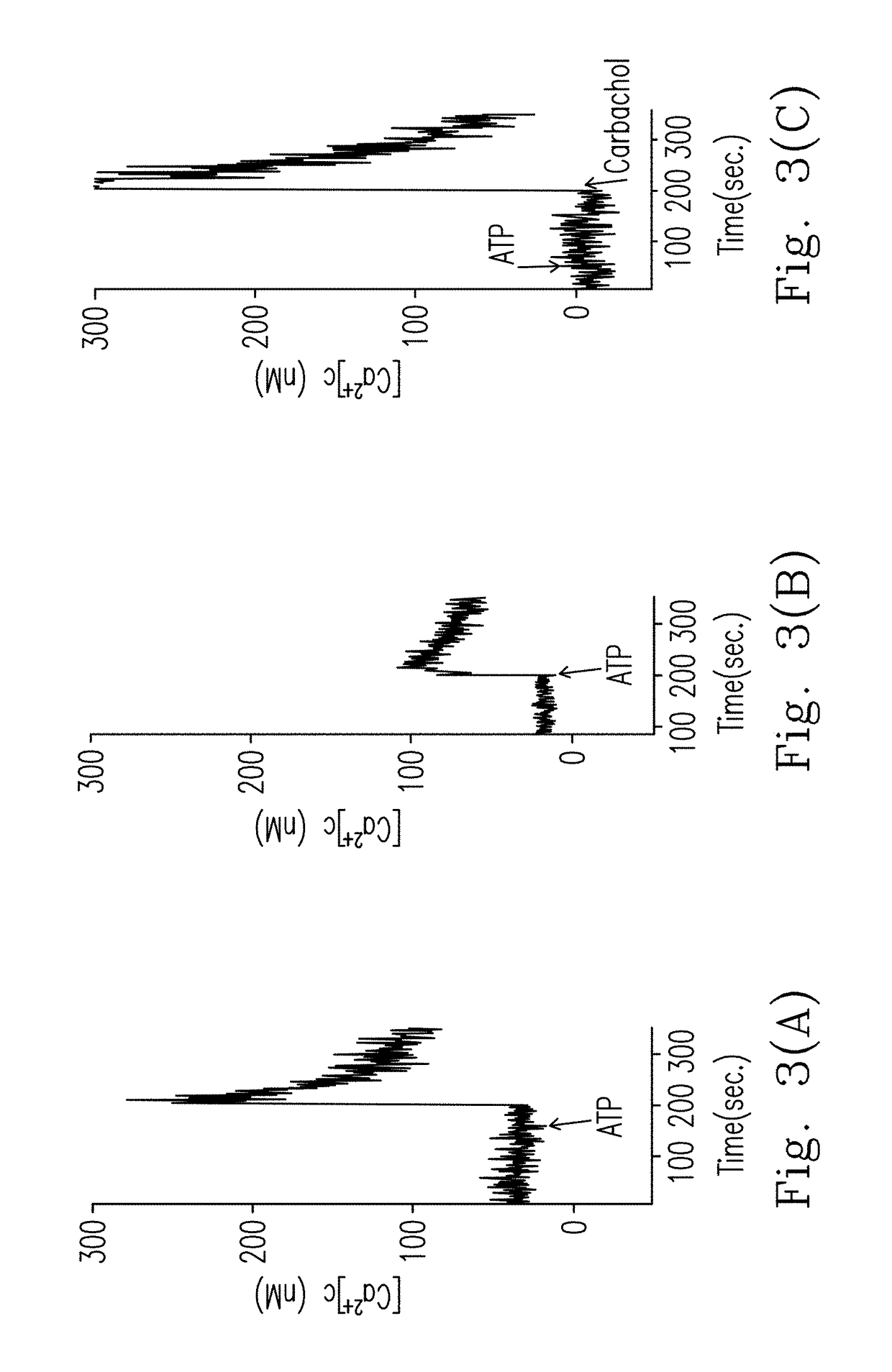
hment and Analysis of the Cell Model for P2R
[0096]1. The incubation of cells:
[0097]Three cell lines, i.e. rat pheochromocytoma cell line PC12, human neuroblastoma cell line SH-SY5Y and human osteosarcoma cell line HOS, were used. PC12 cells were incubated in a Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% (v / v) horse serum (HS) and 5% (v / v) fetal bovine serum (FBS) (Kao et al., Toxicol. Sci., 2012, 125(2):462-472). Human neuroblastoma SH-SY5Y cells were incubated in a DMEM / F-12 medium supplemented with 10% (v / v) FBS (Liu et al., J. Toxicol. Sci., 2009, 34(3):255-263). HOS cells were incubated in a minimum essential medium (MEM) (containing Earle's salts, 2 mM L-glutamine and 0.1 mM non-essential amino acids) supplemented with 1.5 g / L sodium bicarbonate and 1 mM pyruvate sodium, and an extra 5% (v / v) FBS was added during use. Cells were grown in a 5% CO2 humidified incubator at 37° C. The medium was replaced every 2-3 days, and cells were sub-cultured using trypsin-et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| excitation wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com