Skull implanted electrode assembly for brain stimulation
a brain stimulation and implanted electrode technology, applied in external electrodes, sensors, artificial respiration, etc., can solve the problems of headache, muscle aches, headache, and seizure that lasts generally less than one minute,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
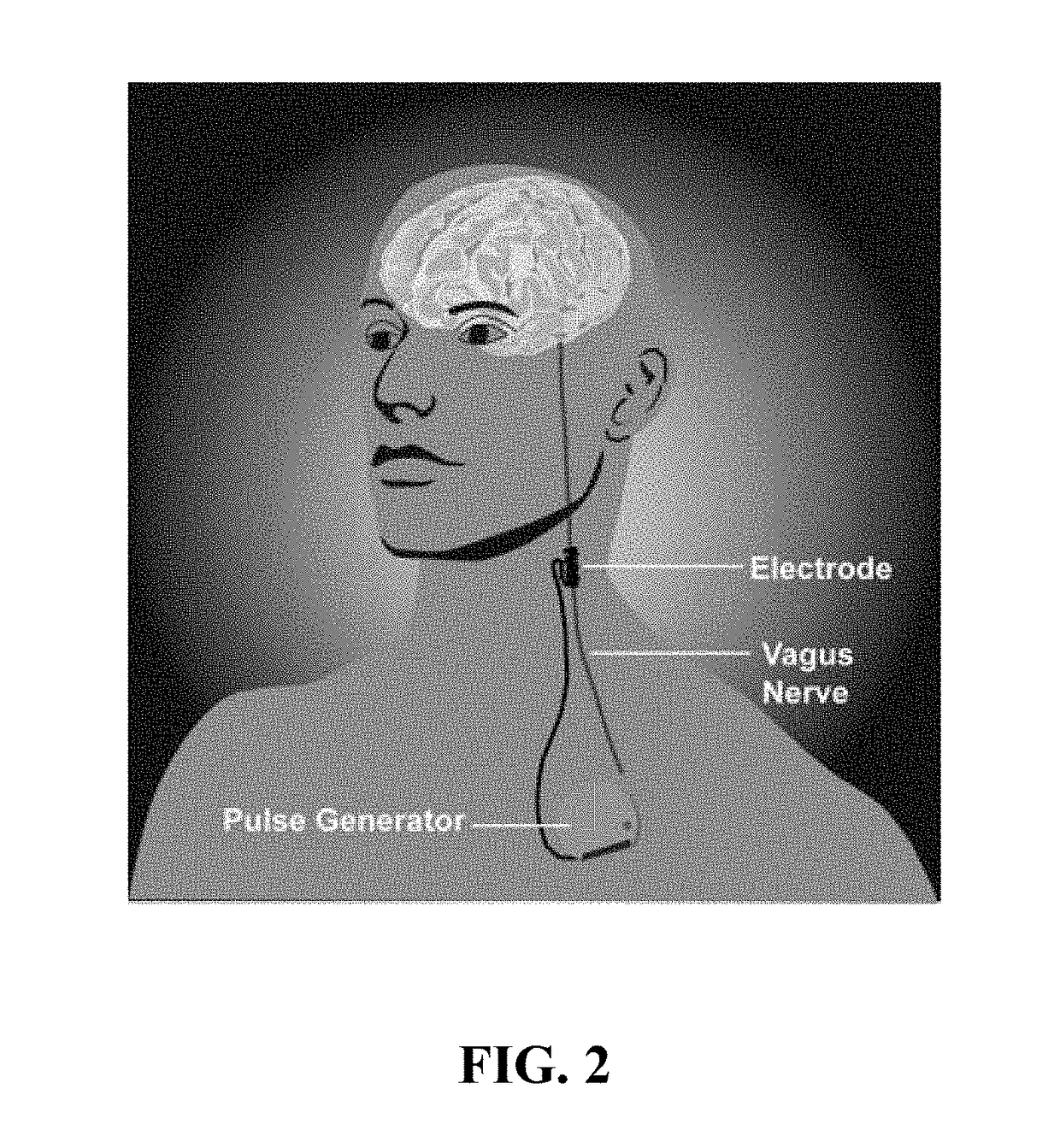
[0091]Embodiments of the present disclosure will now be described more fully hereinafter with reference to the accompanying drawings, in which some, but not all embodiments of the disclosure are shown.
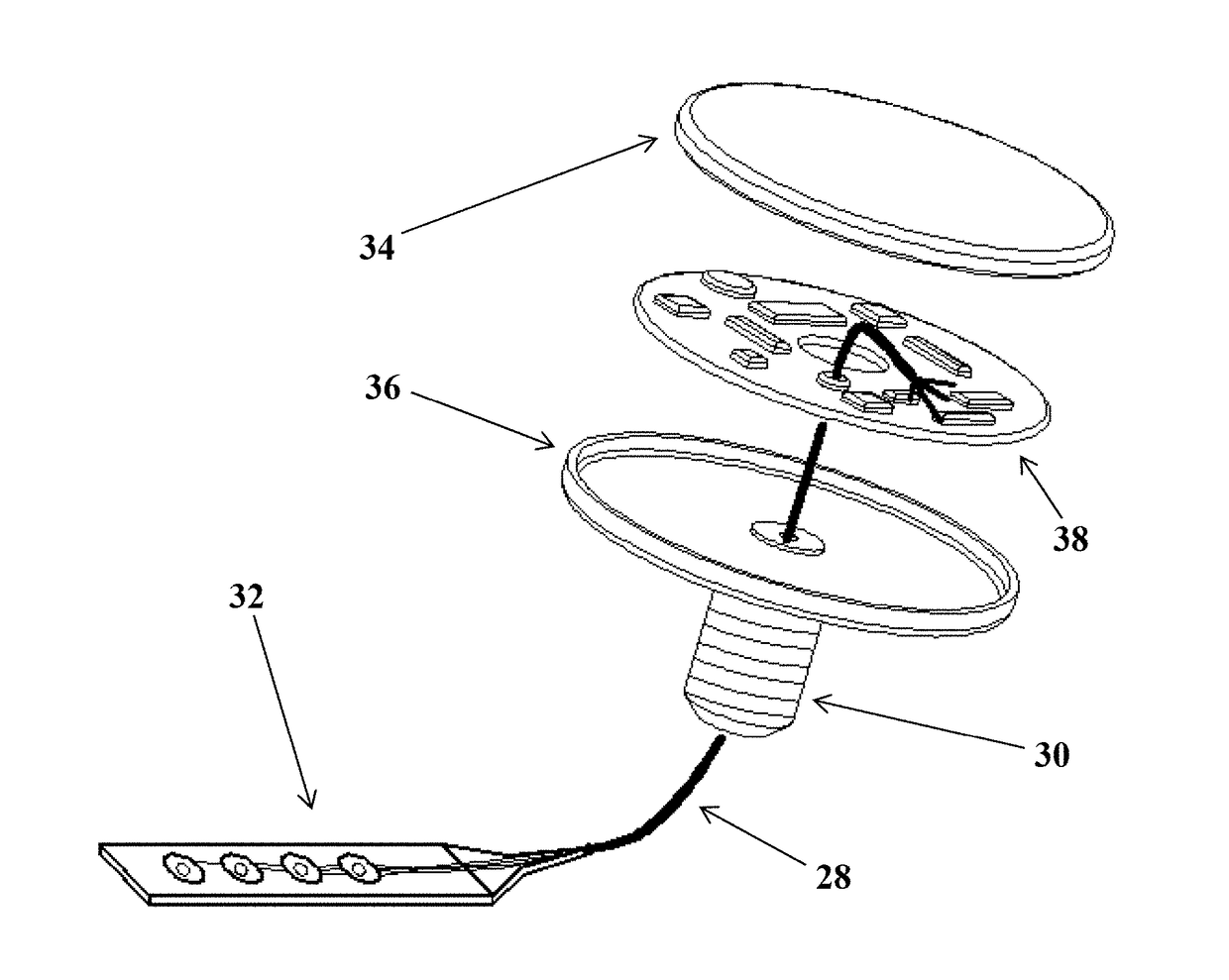
[0092]According to a first aspect, the present disclosure relates to an implantable electrode assembly that comprises an electrically-conductive cannulated skull screw with a head end and a point end. This skull screw is configured to transverse a patient's skull with the point end at the interior surface of the skull and the head end at the exterior surface of the skull. The implantable electrode assembly also comprises an electronics module that has a battery, a computer chip, and a casing, with the electronics module being electrically connected to the head end of the skull screw. The implantable electrode assembly also comprises an electrode housed in an insulated conduit and threaded through the skull screw. The electrode has a connection end and an electrode end, with the connect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com