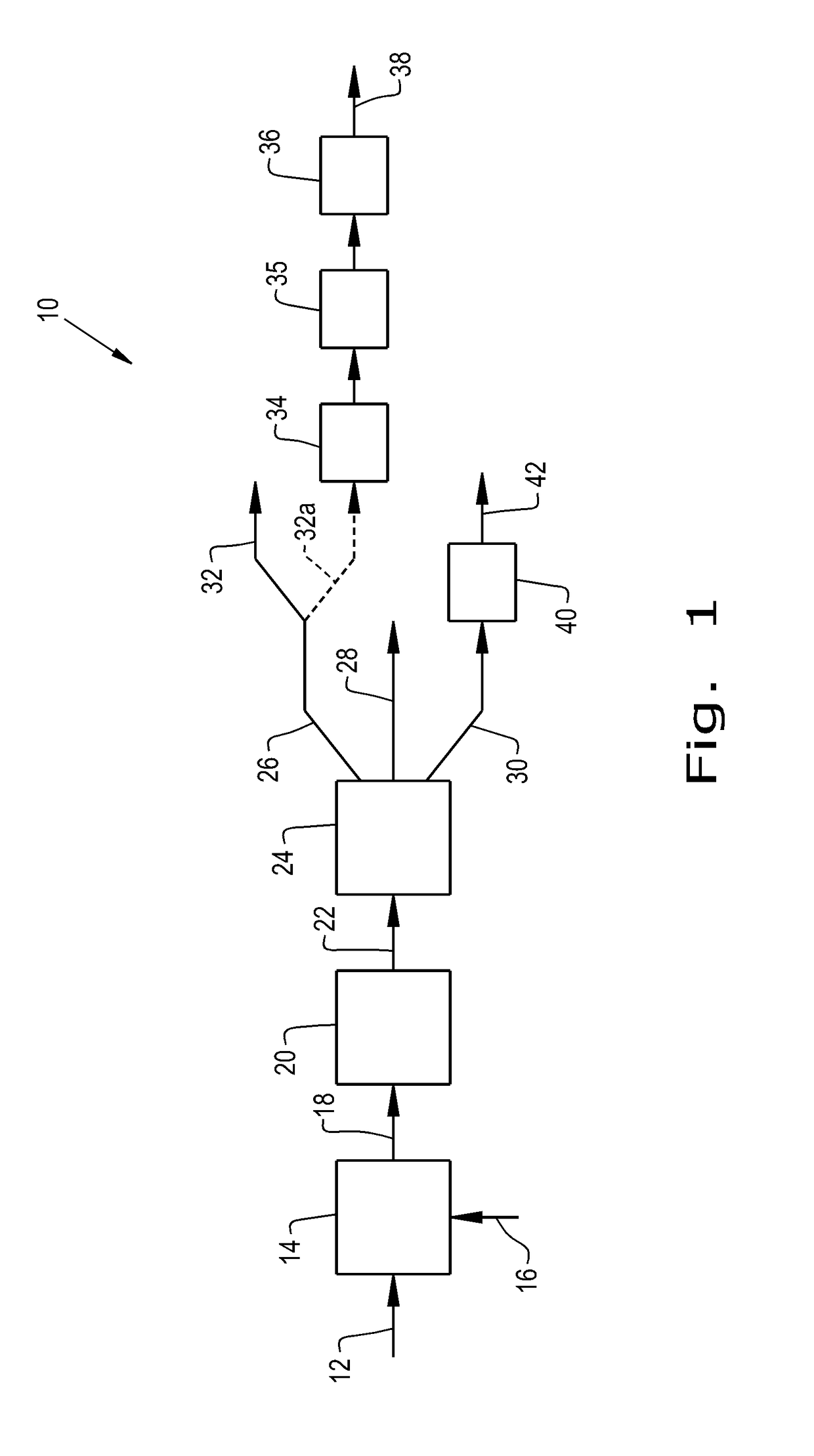
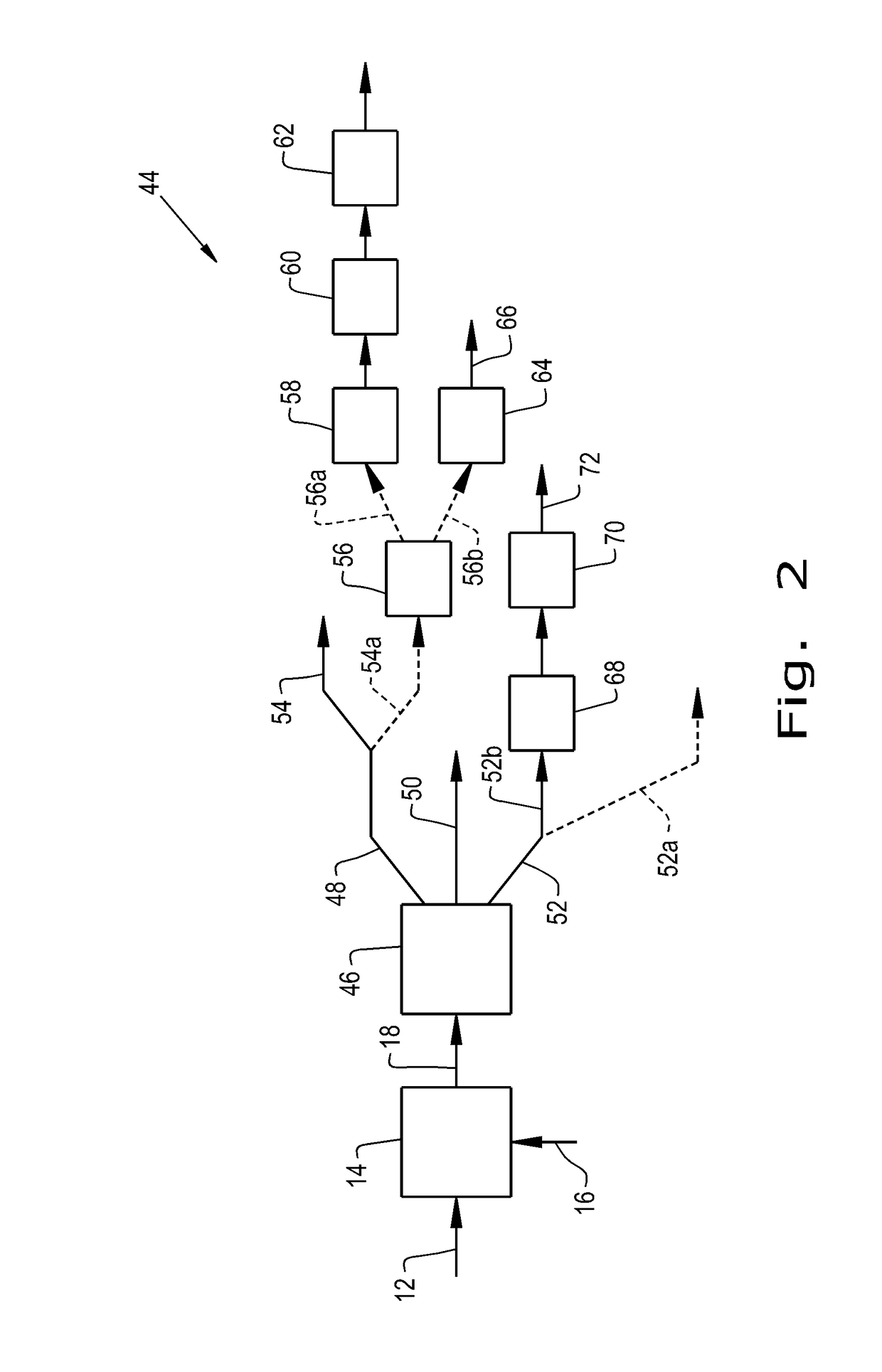
Process for making isoidide
a technology of isoidide and endo hydroxyl group, which is applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, organic chemistry, etc., can solve the problems of amorphous polymerization, asymmetric reactivity and amorphous polymers, and the epimer isomannide, which has two endo hydroxyl groups, and is not currently manufactured on a commercial scal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0066]A ruthenium on carbon catalyst including 2 percent of ruthenium based on the total weight of the catalyst was loaded into a 30 cubic centimeter fixed bed reactor, and hydrogen was thereafter supplied to the reactor at 11.0 MPa, gauge (1600 pounds per square inch, gauge), at a rate of 0.4 liters per minute, together with a liquid feed comprised of 20% dextrose and the remainder of water. The reactor temperature was 130 degrees Celsius, and the liquid hourly space velocity was 1 hr−1.
[0067]The process was continuously run over a period of two weeks, with product samples being drawn on a number of consecutive days within that timeframe. All amounts are reported as percent by total weight.
TABLE 1MANNITOL / DULCITOL / SORBITOLProductsARABITOL %XYLITOL %%IDITOL %Ru / C5.9861.38215.7854.91
example 5
[0070]A sulfided ruthenium on carbon catalyst including 2 percent of ruthenium and 1 percent of sulfur based on the total weight of the catalyst was loaded into a 30 cubic centimeter fixed bed reactor, and hydrogen was thereafter supplied to the reactor at 12.4 MPa, gauge (1800 pounds per square inch, gauge) at a rate of 0.4 liters per minute, together with a liquid feed comprised of 25% dextrose and the remainder of water. The reactor temperature was 170 degrees Celsius, and the liquid hourly space velocity was 0.7 hr−1.
[0071]The process was continuously run over a period of two weeks, with product samples being drawn on a number of consecutive days within that timeframe. All amounts are reported as percent by total weight, with the balance being water.
TABLE 3SampleErythritolxylitolArabitolMannitolSorbitolIditolId%%%%%%RuS / C0.431.262.034.2110.447.29
examples 6-9
[0072]A sponge nickel catalyst was loaded into a 30 cubic centimeter fixed bed reactor, and hydrogen was thereafter supplied to the reactor at 12.4 MPa, gauge (1800 pounds per square inch, gauge), at a rate of 0.4 liters per minute, together with a liquid feed comprised of dextrose, a sorbitol / dextrose mixture, fructose or mannitol and the remainder of water. The reactor temperature was 150 degrees Celsius, and the liquid hourly space velocity was 1 hr−1.
[0073]The process was continuously run over a period of two weeks, with product samples being drawn on a number of consecutive days within that timeframe. All amounts are reported as percent by total weight, with the balance being water.
TABLE 4FeedMannitolSorbitolDulcitolIditol20% dextrose4.0710.390.823.1220% fructose7.769.2702.0020% dextrose / 20%5.8628.1804.99sorbitol14% mannitol7.204.740.611.36
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com