Method to direct differentiation of pluripotent stem cells into functional heart muscle
a technology of pluripotent stem cells and heart muscle, applied in the direction of skeletal/connective tissue cells, biochemistry apparatus and processes, embryonic cells, etc., can solve the problems of large increases in extracellular matrix (ecm), difficult extrapolation, and tissue engineering may be considered an inefficient process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
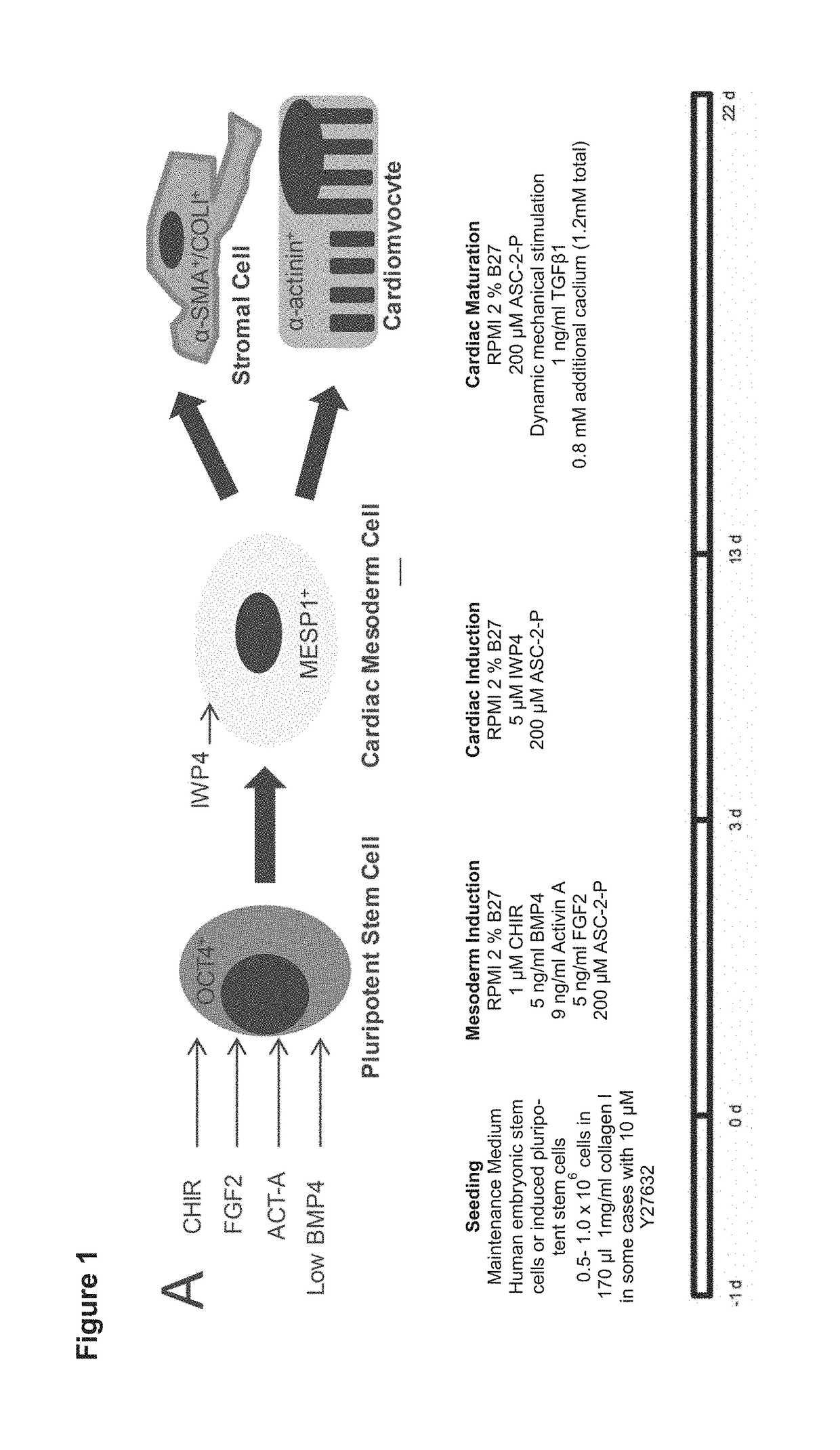
[0145]Cardiac Differentiation Requires Optimization of Early Cardiac Mesoderm Induction
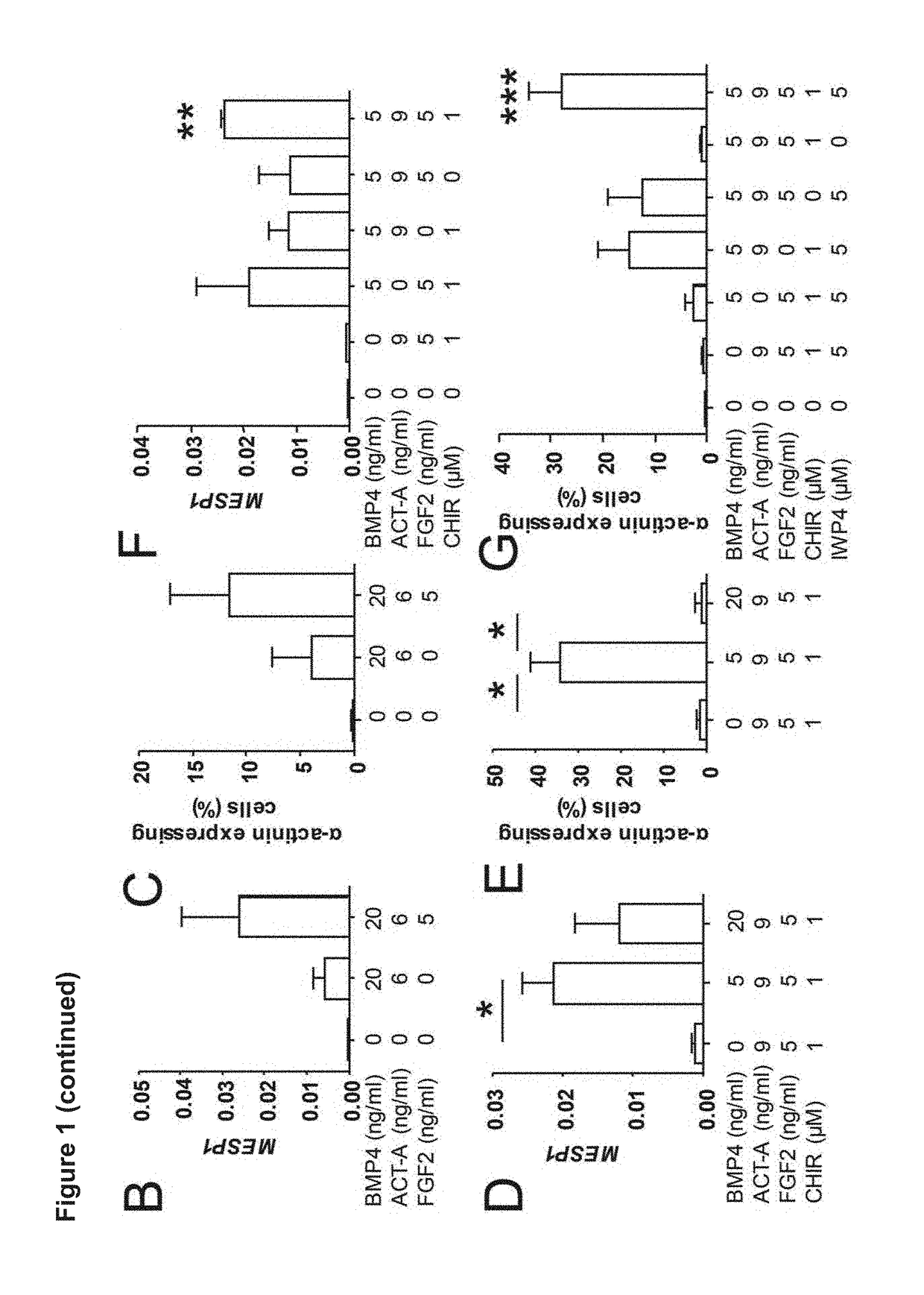
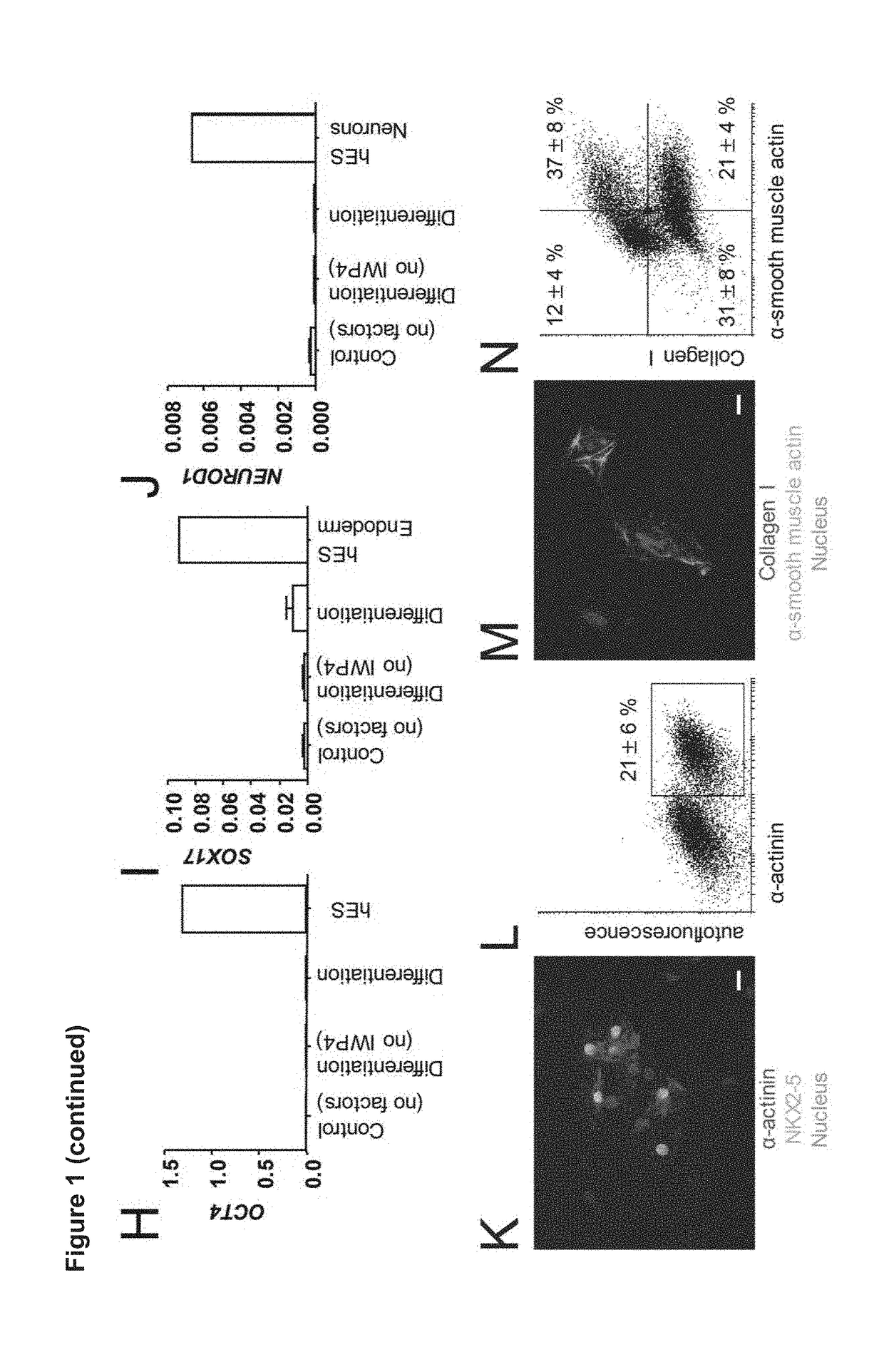
[0146]It has been demonstrated that non-myocyte cell fractions or stromal cells are essential for the function of engineered heart tissues. For this reason a cardiac differentiation protocol was firstly required which consistently produced cardiomyocytes and fibroblasts / stromal cells. The inventors optimized their cardiac differentiation protocol (FIG. 1a) for both yield and consistency, based on a previously published serum-free 2D hPSC differentiation protocol (Hudson et al. Stem Cells Dev 21, 1513-1523 (2012)). It was reasoned that robustness and efficiency could be enhanced if WNT activity was stabilized during the mesoderm induction phase. As surrogate marker for mesoderm induction MESP1 expression was analysed by qPCR on culture day 3; this was followed by flow cytometry for α-actinin (cardiomyocyte marker) at day 16, which were found correlated very well with the amount of beating activity....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com