Methods for treating muscle wasting and bone disease using novel hybrid actriib ligand trap proteins
a technology of ligand trap proteins and muscle wasting, which is applied in the direction of peptide/protein ingredients, immunological disorders, metabolism disorders, etc., can solve the problems of robbery of patient mobility and independence, bone diseases can be very painful, and bone diseases can be very serious, so as to prevent muscle wasting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0298]The polypeptides of the present disclosure can be prepared according to recombinant DNA techniques that are well known to those of skill in the art. In this example, the preparation of the hybrid soluble ActRIIB-ECD polypeptides is generally described.
[0299]Various hybrid ActRIIB-ECD polypeptides were designed by substituting multiple amino acid residues at selective positions within the human ActRIIB extracellular domain with amino acid residues derived from the human ActRIIA extracellular domain at corresponding positions based on sequence alignment at the amino acid level. DNA expression cassettes encoding the hybrid ActRIIB-ECD polypeptides were generated by using site-directed mutagenesis and subsequently engineered into Fc fusion protein constructs by placing in frame a cDNA fragment encoding human immunoglobulin light chain signal peptide at the 5′ end and a DNA fragment encoding a peptide linker followed by human Fc at the 3′ end.
example 2
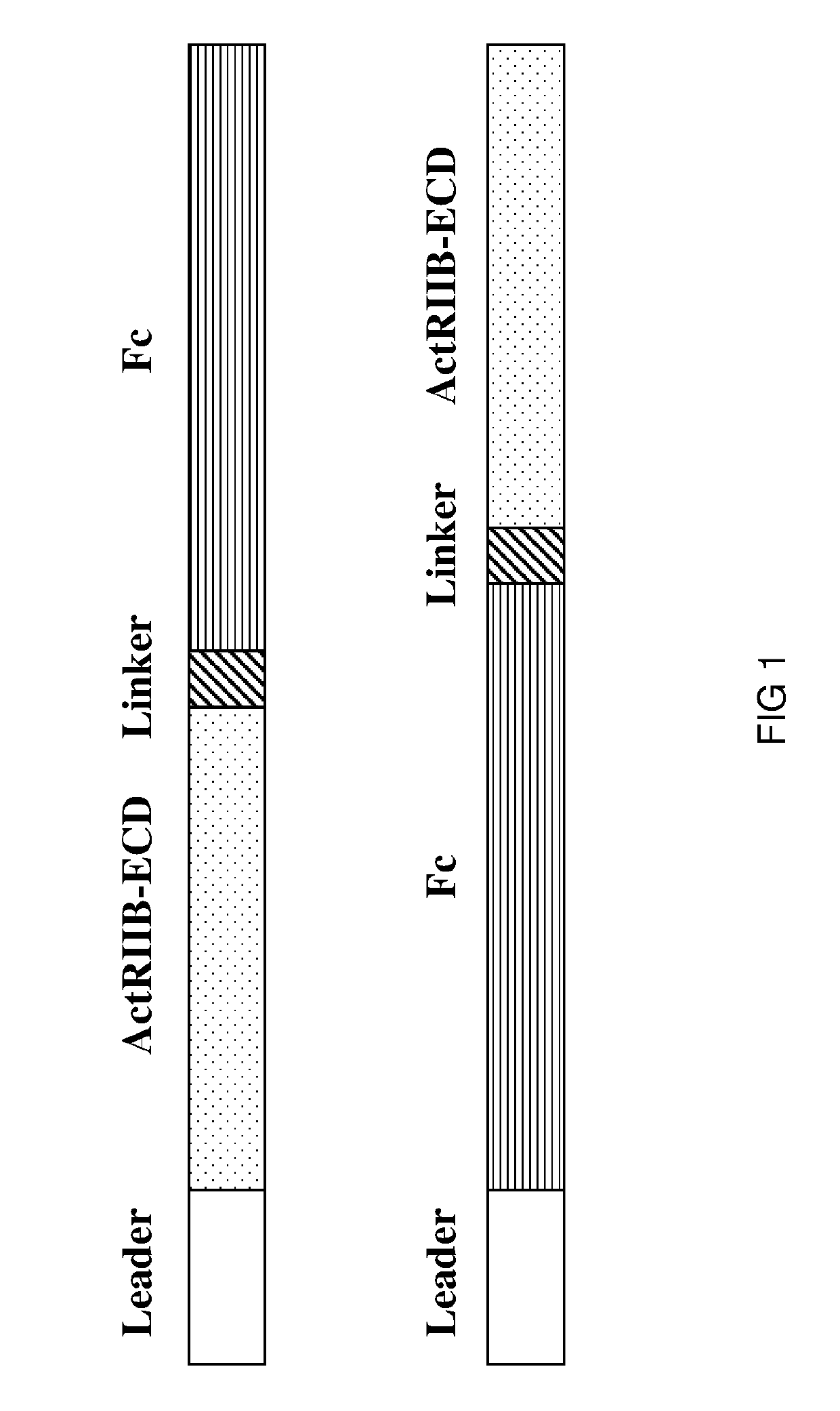
[0300]In this example, the preparation of the hybrid ActRIIB ligand trap proteins configured as depicted in FIG. 1 is generally described.
[0301]Synthetic DNA cassettes encoding various hybrid ActRIIB ligand trap proteins, each containing a signal peptide leader sequence (SEQ ID NO: 49 or 50), a hybrid soluble ActRIIB-ECD polypeptide from Example 1 (or a wild-type ActRIIB-ECD sequence), a peptide linker sequence (SEQ ID NO: 44), a hinge linker sequence (SEQ ID NO: 118) and an Fc domain sequence (SEQ ID NO: 39 or 41 or 43), are cloned into Freedom pCHO 1.0 and pcDNA3.1 expression vectors (Life Technologies).
[0302]For stable transfection, the pCHO 1.0 expression vectors encoding the various hybrid ActRIIB ligand trap proteins were each transfected in CHO-S cells using FreeStyle MAX Reagent (Life Technologies). 48 hours after transfection, the cells were grown in serum-free CD FortiCHO medium containing puromycin and methotrexate (MTX) selection for 3-7 weeks at 37° C. in a shaker CO2 i...
example 3
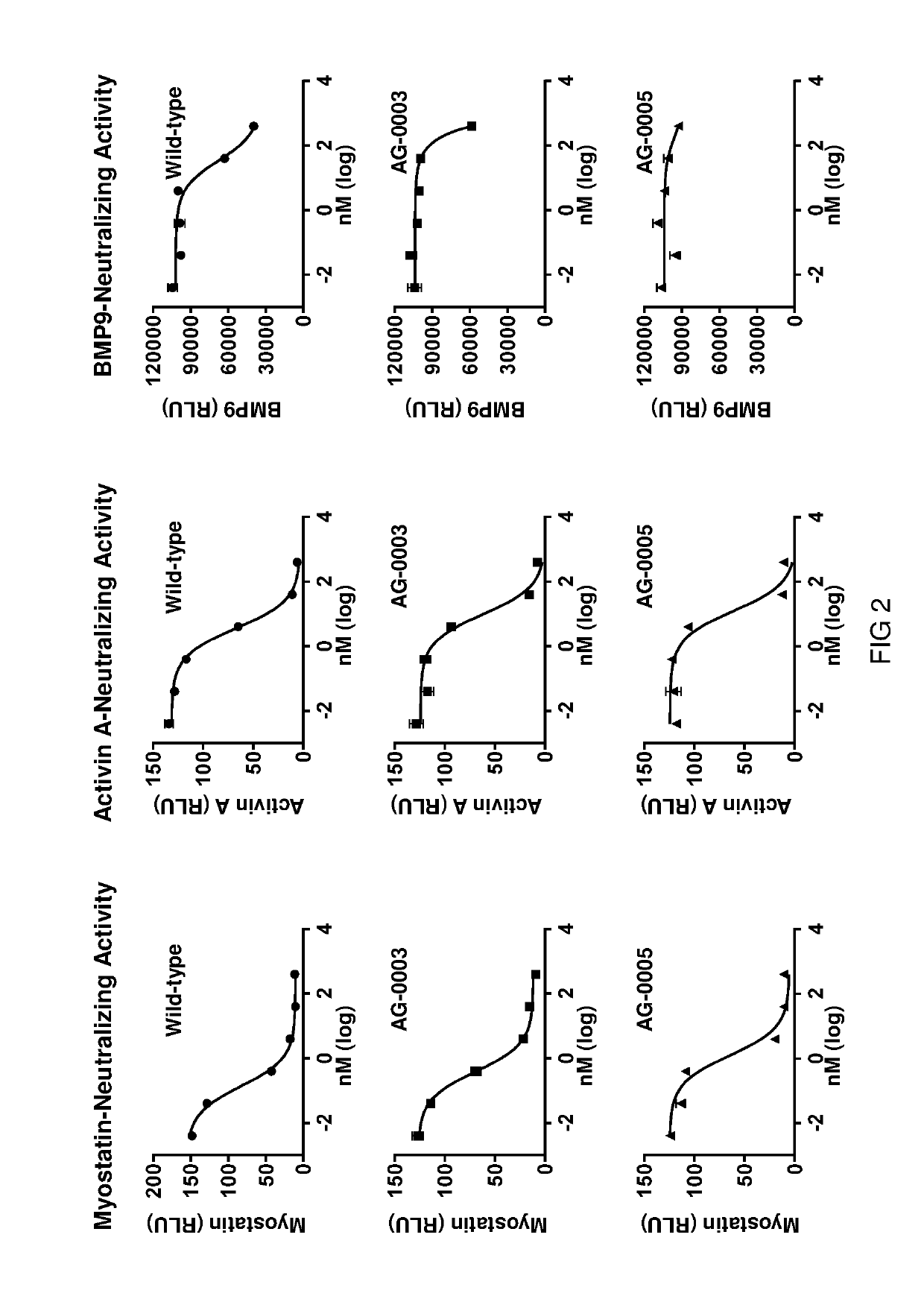
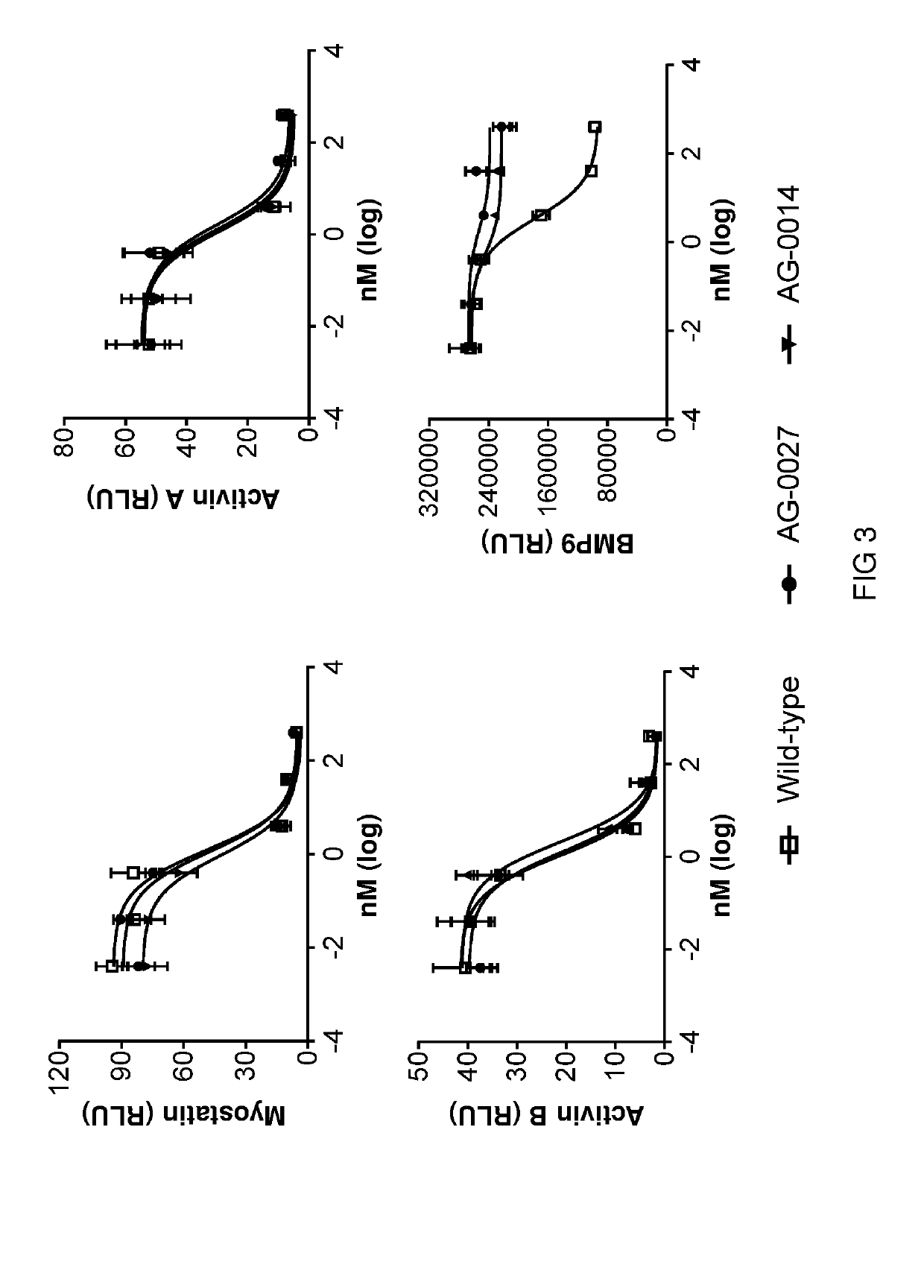
[0305]In this example, the myostatin and BMP9 binding activities of seven of the hybrid ActRIIB ligand trap proteins is evaluated.
[0306]Myostatin and BMP9 binding activities of various hybrid ActRIIB ligand trap proteins were initially analyzed using Octect Red (ForteBio). Purified proteins or conditioned media were individually loaded to AHC biosensors with maximum loading. Following a baseline washing phase, the sensors were exposed to 10 nM myostatin or BMP9, respectively, for an association step followed by a dissociation step. All experiments were performed with shaking at 1,000 rpm. Binding activity was analyzed using ForteBio's software with KD being calculated using the ratio Kd / Ka.
Results
[0307]Hybrid ActRIIB ligand trap proteins were examined in comparison with the wild-type ActRIIB-ECD-Fc fusion protein for binding activities against myostatin and BMP9. The results indicate that the hybrid ActRIIB ligand trap proteins exhibit a marked reduction in binding affinity to BMP9 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| trabecular thickness | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| W/W | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com