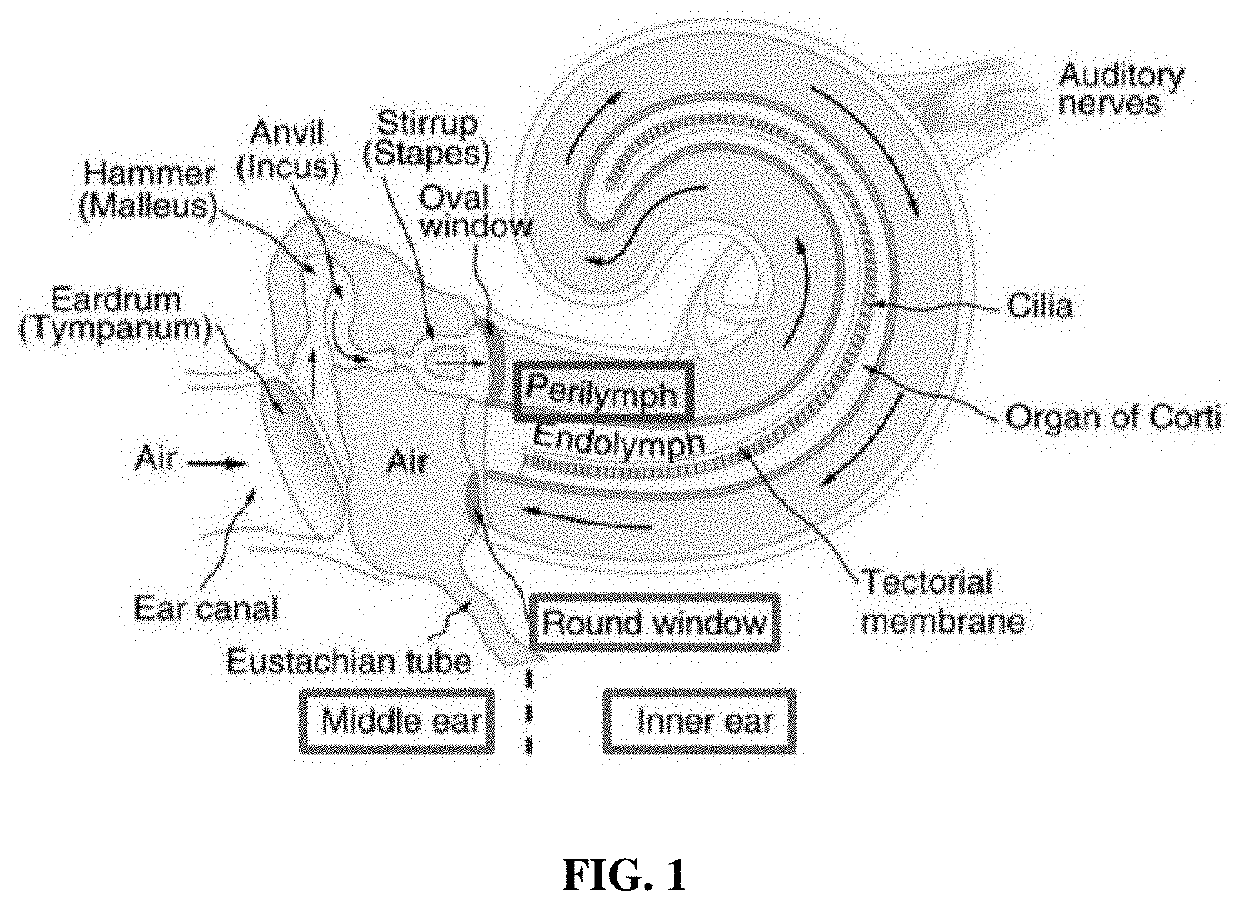
Apoptosis inhibitor formulations for prevention of hearing loss
a technology of apoptosis inhibitors and formulations, applied in the direction of sense disorder, organic active ingredients, drug compositions, etc., can solve the problems of irreversible hearing loss, incidence and severity of ototoxicity, restricted social life, etc., to prevent pathological and/or physiological conditions, prevent ototoxicity, and maintain the release of apoptosis inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Hydrogel for Loading 2-(4-(2,4-dichlorophenethyl)-3,6-dioxo-1-(2-(thiophen-2-yl)ethyl)piperazin-2-yl)-N-(2-(5-methoxy-1H-indol-3-yl)ethyl)acetamide (LPT99)
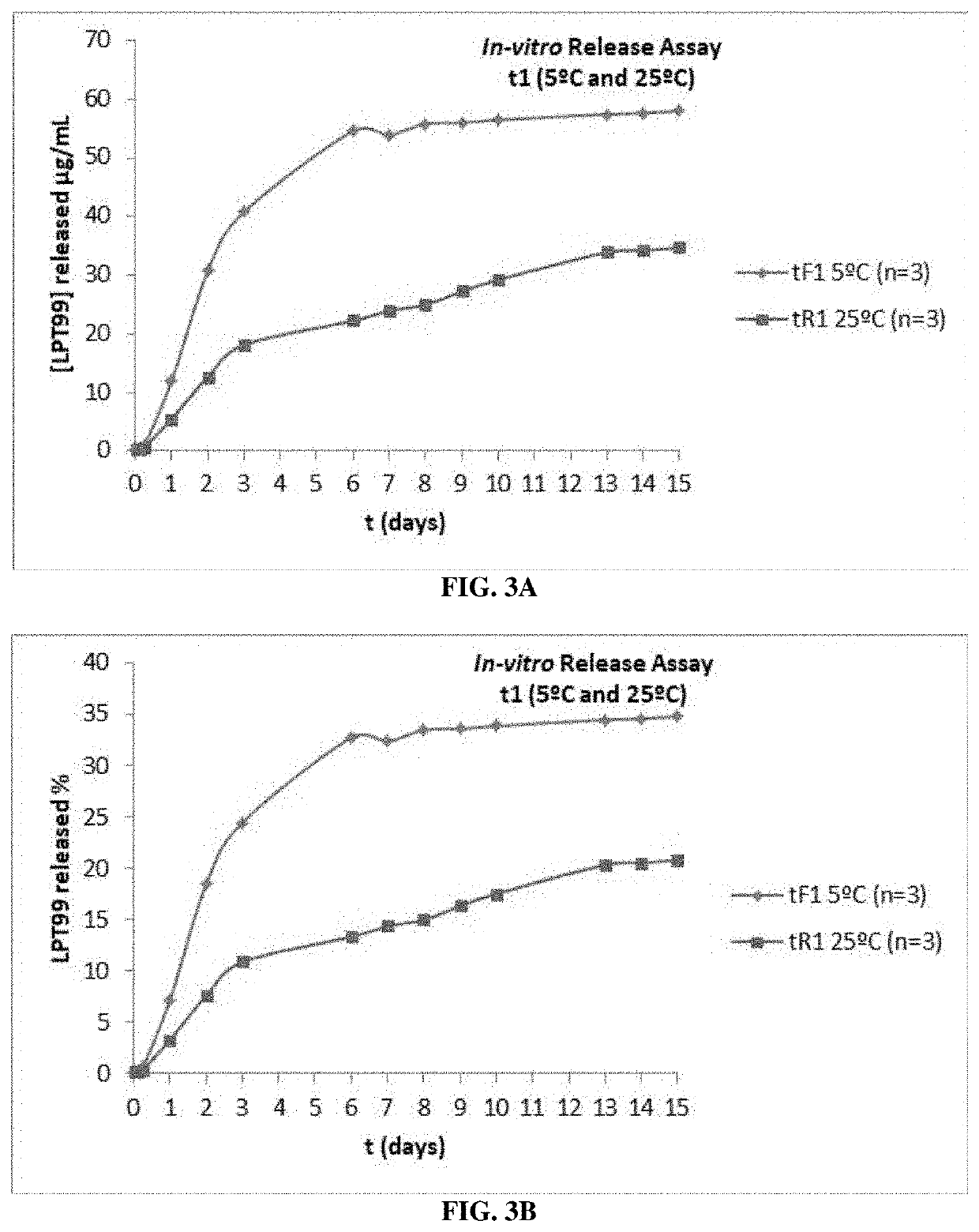
[0165]In vitro experiments with LPT99 demonstrated its specificity for Apaf-1, resulting in inhibition of apoptotic protease activating factor-1 (Apaf-1). In a cellular model of CisPt-induced apoptosis, LPT99-treated cells showed a decreased release of Cytc from mitochondria, reduced caspase-3 activation, and an improved cell viability, evidence of the cytoprotective effect of LPT99 (Cervantes, et al., IEB Symposium, Montpellier, Abstract P77, “Inhibition of APAF-1 with LPT99 prevents cisplatin-induced apoptosis in HEI-OCl auditory cells”, Sep. 18, 2016; Maurillo-Cuesta, et al., IEB Symposium, Montpellier, Abstract P78, Inhibition of Apaf-1 with LPT99 prevents cisplatin-induced hearing loss, Sep. 18, 2016).
[0166]These studies showed that the compound LPT99 could be effective in preventing hearing loss due to exposure to cisp...
example 2
of the Final Product
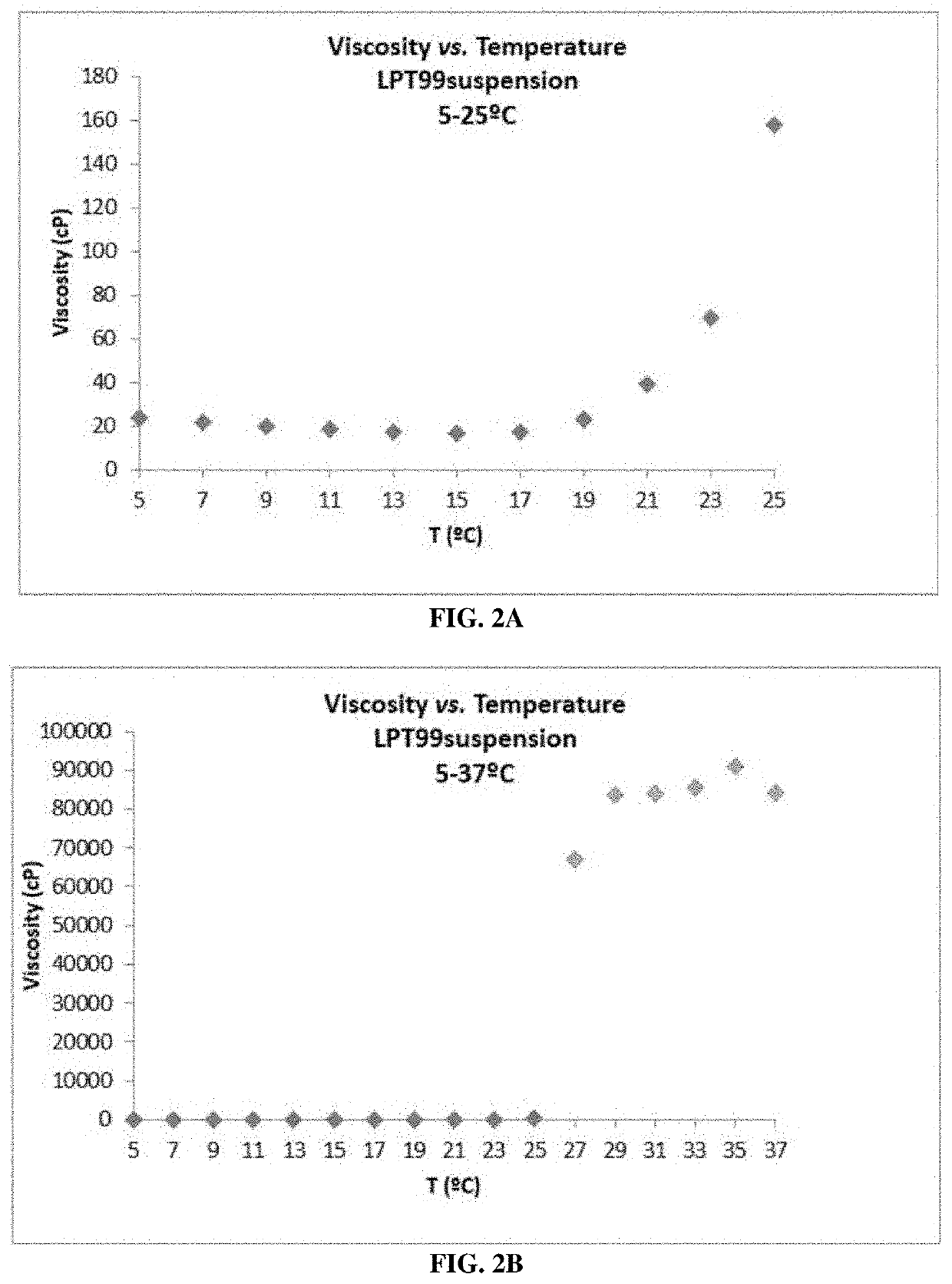
[0182]The viscosity measurement of the hydrogel was performed to determine the behavior of the viscoelastic agent once gelled within the ear. The measurement was carried out following the European Pharmacopoeia Method, section 2.2.10 (measured at 37° C., body temperature).
[0183]P407 is a thermoreversible compound, existing in a liquid or gel state depending on its temperature. Accordingly, it can form a semi-solid gel at body temperature of 37° C., being liquid at room temperature. During the development process, this allowed formation of an easy to handle solution with the LPT99 molecule, which gels at 37° C., once the solution is administered to a patient.
[0184]Viscosity measurements were performed at 37° C. to simulate the real conditions of application of the gel, once it has gelled. The solution was first placed in a climatic chamber at 37° C. to gel (20 mL of solution for about 1 h), and once gelled, the viscosity was measured by maintaining this temperatur...
example 3
Studies Showing Efficacy of Formulation
[0211]Materials and Methods
[0212]The specificity of LPT99 was tested in vitro on Apaf-1, caspase 3, and caspase 9 (proteins from the apoptotic cascade), and a broad panel of potential pharmacological targets.
[0213]To identify off-target activities of LPT99, its selectivity against a panel of receptors (44 G-protein-coupled receptors [GPCR] and 4 non-GPCR), 4 ion channels, and 3 transporters were analyzed. The cell line, HEI-OCl (house ear institute organ of Corti 1), which expresses several characteristic markers of the organ of Corti sensory cells (Kalinec, et al., Audiol. Neurootol. 2003, 8(4), 177-89), was used to evaluate the efficacy of LPT99 in preventing apoptosis due to CisPt prevention. Cells were pre-incubated with LPT99, followed by CisPt prevention for 24 hours. Under these conditions, the 50% inhibitory concentration (IC50) for caspase 3 was 5.2±1.6 μM. Both LPT99 stereoisomers were equally effective and equivalent to the racemic m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com