Detection of immune checkpoint molecules by deglycosylation
a technology of glycosylation and immune checkpoint, which is applied in the field of molecular biology, can solve the problems of inability to use pd-l1 as a predictive biomarker in nsclc, and the inability to direct biomarkers, so as to increase the expression and increase the level of said immune checkpoint protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ection Method
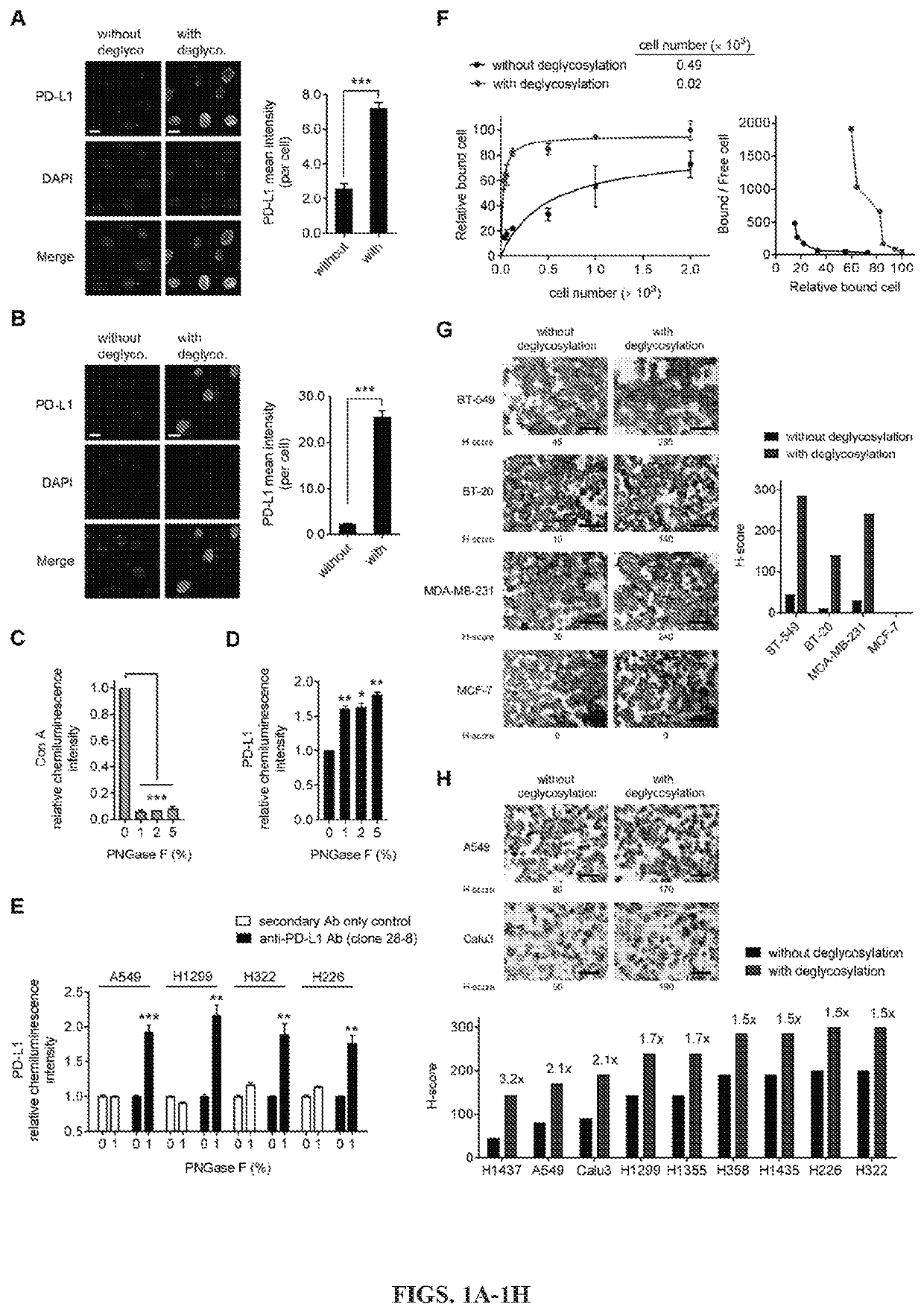
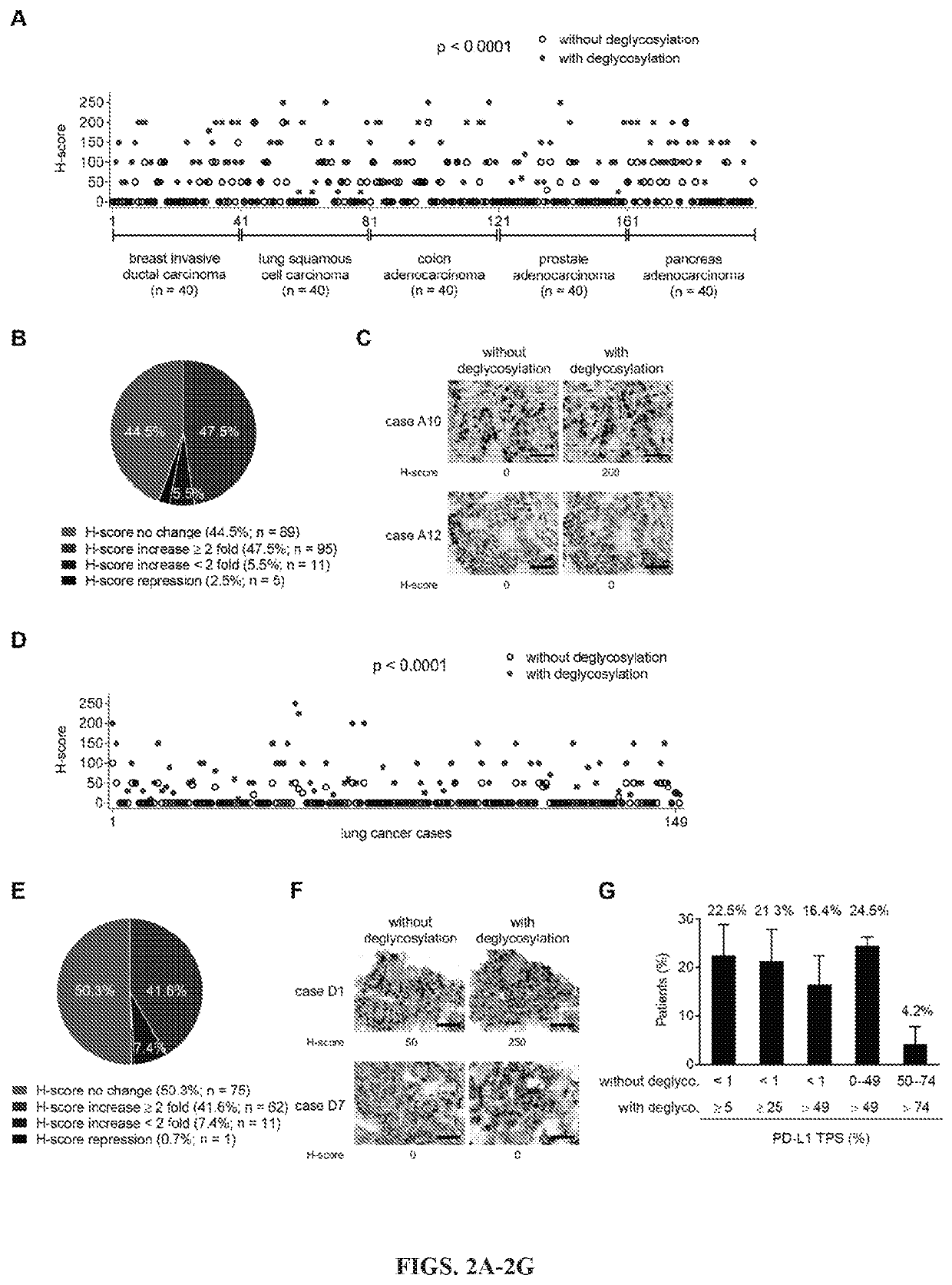
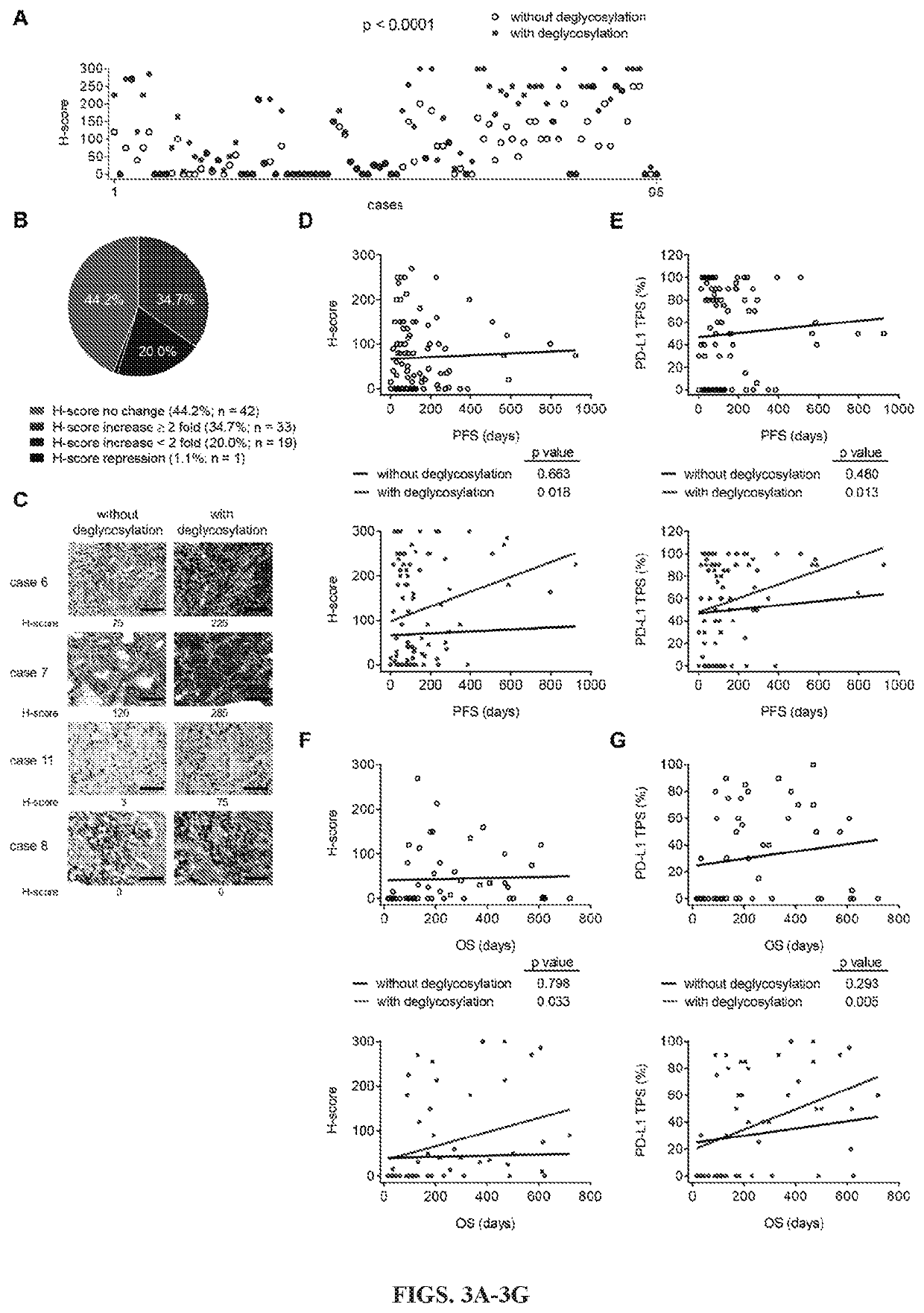
[0125]Removal of N-linked glycosylation enhances anti-PD-L1 signal in human cancer cells: The migration pattern of PD-L1 on gel electrophoresis was heterogeneous as illustrated by a range of bands at ˜50 kDa with heavy glycosylation in a panel of human lung and basal-like breast cancer (BLBC) but not non-BLBC cell lines (FIGS. 6A and 6B). Treatment with recombinant glycosidase (peptide-N-glycosidase F; PNGase F) to remove the entire N-linked glycosylation (deglycosylation, herein after) resulted in a homogenous pattern of PD-L1 at ˜33 kDa (FIGS. 6A and 6C). To determine whether the N-linked glycan structure of PD-L1 hinders antibody-based detection targeting the PD-L1 antigen, cells were first pretreated with or without PNGase F followed by immunofluorescence confocal microscopy analysis. The fluorescent intensity of PD-L1 was significantly enhanced after PNGase F treatment in lung cancer and BT-549 BLBC cells compared with no treatment (FIGS. 1A, 1B, and 6D). The resul...
example 2
and Methods
[0139]Cell culture: All human cells lines cultured at 37° C. under 5% CO2 were obtained from the American Type Culture Collection (Manassas, Va., USA), including breast cancer (BT-549, BT-20, MDA-MB-231, MCF-7), lung cancer (H1437, A549, Calu3, H1299, H1355, H358, H1435, H226, H322), and immune (Jurkat T lymphocytes, THP1 monocytes) cell lines. Human breast cancer cell lines (BT-549, BT-20, MDA-MB-231, MCF-7) and H1435 cells are female-derived cell lines; other cell lines used are male-derived cells. All cell lines were independently validated by STR DNA fingerprinting at The University of Texas MD Anderson Cancer Center and characterized as mycoplasma negative. BT-549, BT-20, MDA-MB-231, MCF-7, and A549 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) / F12, supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic mixture. Calu3 cells were cultured in Eagle's Minimum Essential Medium, supplemented with 10% FBS and 1% antibiotic mixture. Other cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com