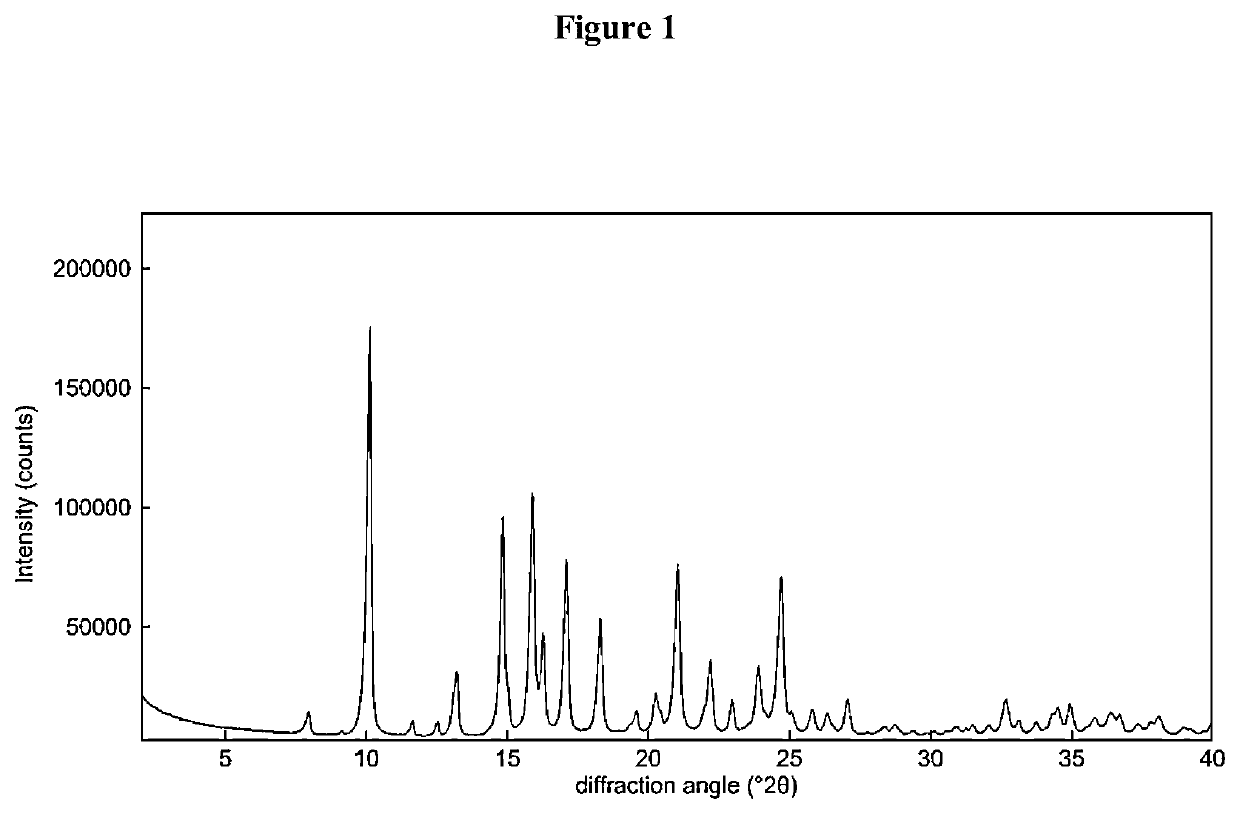
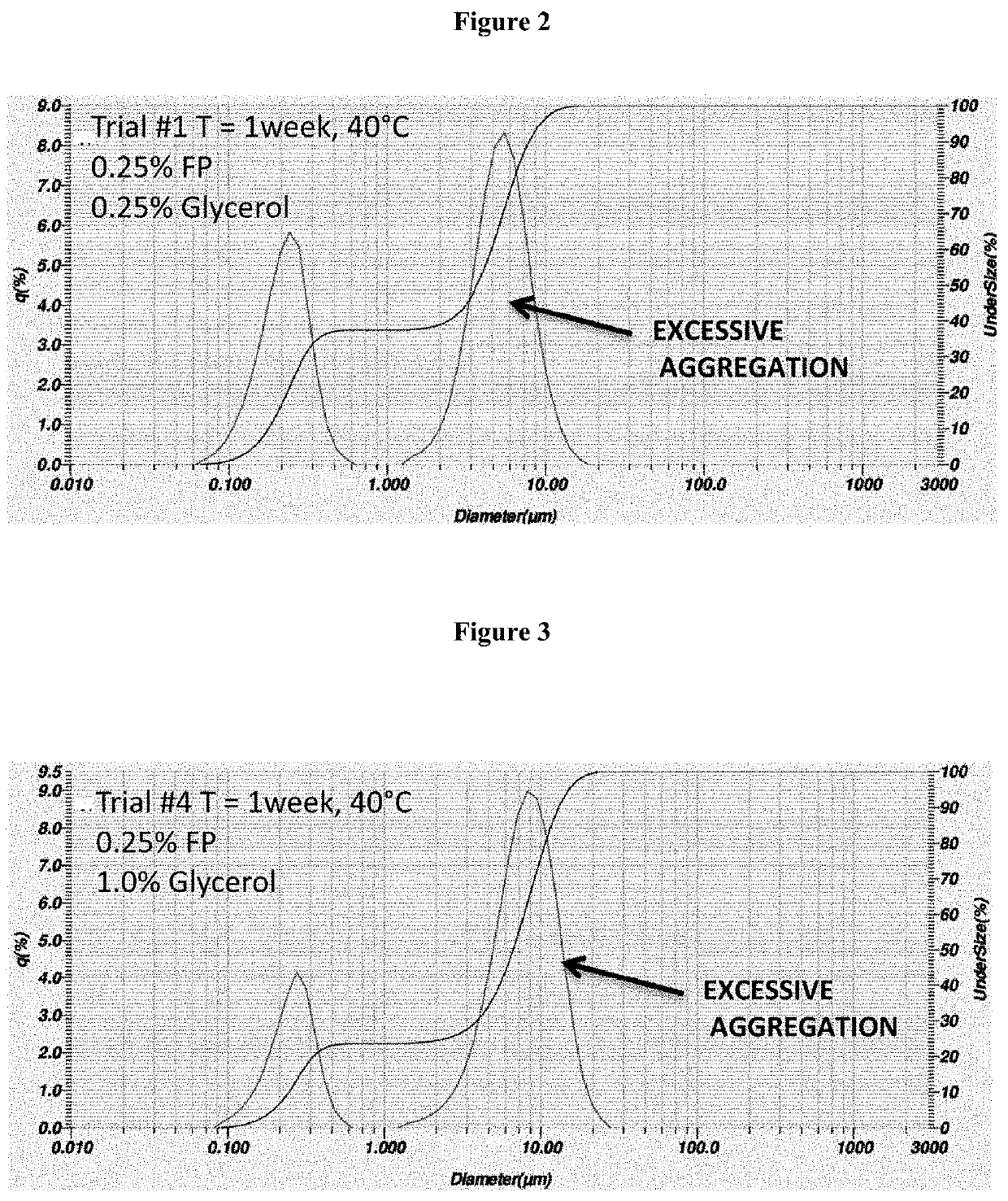
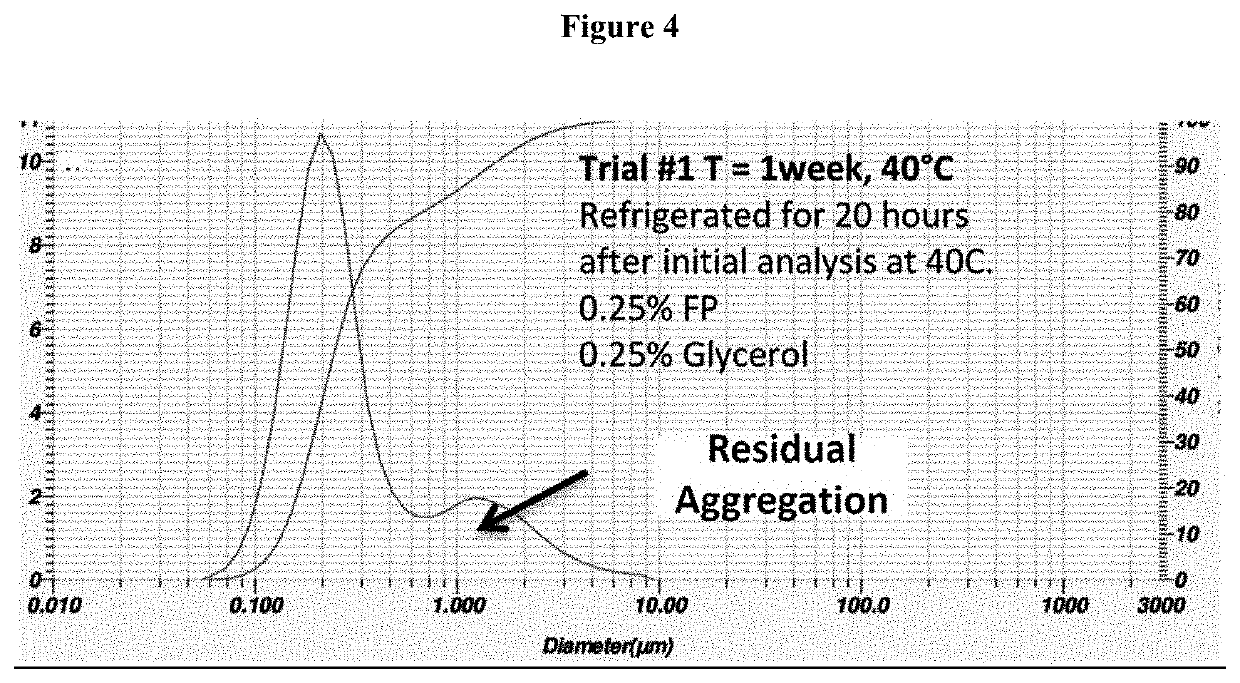
Process for the preparation of sterile ophthalmic aqueous fluticasone propionate form a nanocrystals suspensions
a technology of fluticasone and nanocrystals, which is applied in the field of preparation of sterile topical ophthalmic nanosuspensions, can solve the problems of loss of stabilizing effect, difficult de-agglomeration of nanocrystals, and difficult storage, so as to improve the preservation of multi-dose ophthalmic compositions, stable nanosuspensions, and easy adaptability to large-scale preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0124]Preparation of Nanocrystals of Fluticasone Propionate Form A
[0125]Preparation of Phase I Solution
[0126]In a 2 L process vessel, 106.26 g polysorbate 80 (Tween 80), 963.34 g polypropylene glycol 400 (PPG 400), 324.10 g polyethylene glycol 400 (PEG 400), were added at room temperature and stirred together until all components were dissolved, then 6.3 g fluticasone propionate polymorph 1 was added into the solution and stirred until a clear solution was obtained. The obtained solution was filtered using a 0.8 / 0.2 μm Polyethersulfone (PES) filter and kept refrigerated at 2-8° C. until use.
[0127]Preparation of Phase II Solution
[0128]In an 8 L process vessel an initial quantity of about 5276 g of purified water was added and stirred with an over-head mixer so that a vortex was generated.
[0129]6.01 g polyethylene glycol 40 stearate (PEG-40 stearate), 5.98 g benzalkonium chloride (solution 10%), and 24.02 g methyl cellulose 15 cP were added and stirred till methyl cellulose was comple...
example 2
[0147]Preparation of a Sterile Topical Ophthalmic Aqueous Nanosuspension of Fluticasone Propionate Form A
TABLE 1Fluticasone propionate nanosuspension compositionIngredientsAmount (% w / w)Fluticasone propionate Form A nanocrystals0.10Methylcellulose 4000 cP0.50Polysorbate 800.2Edetate disodium, dihydrate0.10Boric acid1.0Glycerin0.9Benzalkonium chloride0.01Sodium chloride0.055Water for injectionq.s. to 100%pH7.3-7.5
[0148]Step 1) Preparation of Vehicle 1 (Vehicle without Glycerin)
[0149]In a 20 L process vessel, 17600 g water for injection were heated at 80° C., 100.0 g methylcellulose 4000 cP were slowly added and the mixture is stirred until methylcellulose was dissolved.
[0150]The solution was cooled at 40° C., 200.0 g boric acid was added and the pH was adjusted at 7.4 with sodium hydroxide (1N).
[0151]The following excipients were added in the specific following order: 20.0 g edetate disodium dihydrate, 11.0 g sodium chloride, 4.0 g benzalkonium chloride (solution 50%), 40.0 g polysor...
example 3
[0188]Evaluation of the Stability of the Nanosuspension of Example 2
[0189]The nanosuspension composition prepared in example 2 was subjected to storage stability testing by storing the nanosuspension at three different temperatures and humidity conditions (5° C., 25° C. / 40% RH; 40° C. / 25% RH) The resuspendability of the nanosuspension, the content of fluticasone propionate, the particle size distribution and the content of benzalkonium chloride were assessed at 1 month and 3-month time-points.
[0190]The results reported in tables 2 and 3 show that the nanosuspension was physically and chemically stable upon manufacture and storage. No change in physical appearance of the nanosuspension upon storage was noticed. The nanosuspension did not show any sign of chemical degradation as the chemical assay of fluticasone propionate was well within the limit of 90%-110% of the label claim upon storage. The related substances and total impurities remained within the specified limits of not more ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com