Organochromium/metallocene combination catalysts for producing bimodal resins
a technology of bimodal resins and organic compounds, applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of significant challenges in catalyst development, not a single support capable of activating both types of catalysts, etc., to increase the acidity of solid oxides and high surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Fluorided Silica-Alumina Support
[0244]Silica-alumina was obtained from Grace Davison, a division of W.R. Grace and Company, as MS 13-110 containing about 13% alumina and about 87% silica. The silica-alumina had a pore volume of about 1.2 cc / g and a surface area of about 450 m2 / g. The silica-alumina was impregnated with about 0.1 g ammonium bifluoride per gram of the support. The ammonium bifluoride was applied in the form of an aqueous solution. The resulting damp powder was dried for about 16 hours at about 110° C. Ten grams of the fluorided silica-alumina was placed in a 1.75 inch quartz tube fitted with a sintered quartz disk at the bottom. Air or nitrogen, dried by passing through a 13× molecular sieve column, was blown upward through the disk at a linear rate of about 1.6 to about 1.8 standard cubic feet per hour. An electric furnace around the quartz tube was then turned on and the temperature was raised at about 400° C. / hr to 450° C. and held at about 450° ...
example 2
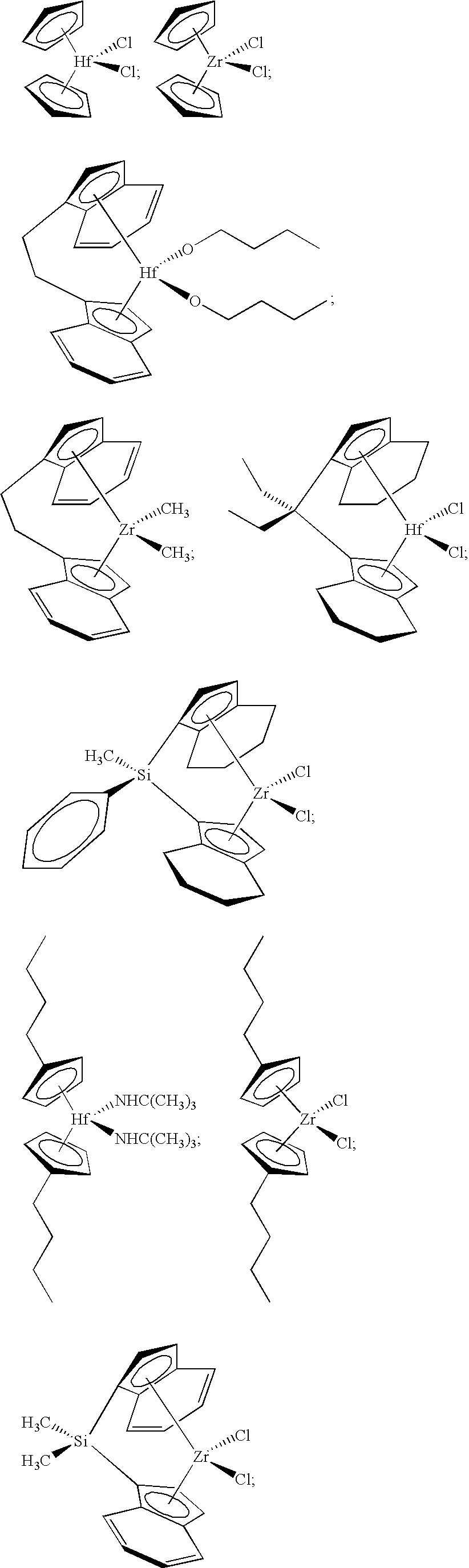
Preparation of the Dicumene Chromium Catalyst
[0245]Fluorided silica-alumina was prepared as described in Example 1. The fluorided silica-alumina was found to have a surface area of about 300 m2 / g and a pore volume of about 1.1 cc / g. Dicumene chromium (0) was impregnated onto this fluorided silica-alumina support from a heptane solution in an amount equal to 1 wt % chromium based on the weight of the support. The heptane was then evaporated off the support under an atmosphere of flowing nitrogen, while the catalyst was warmed to about 50° C.
example 3
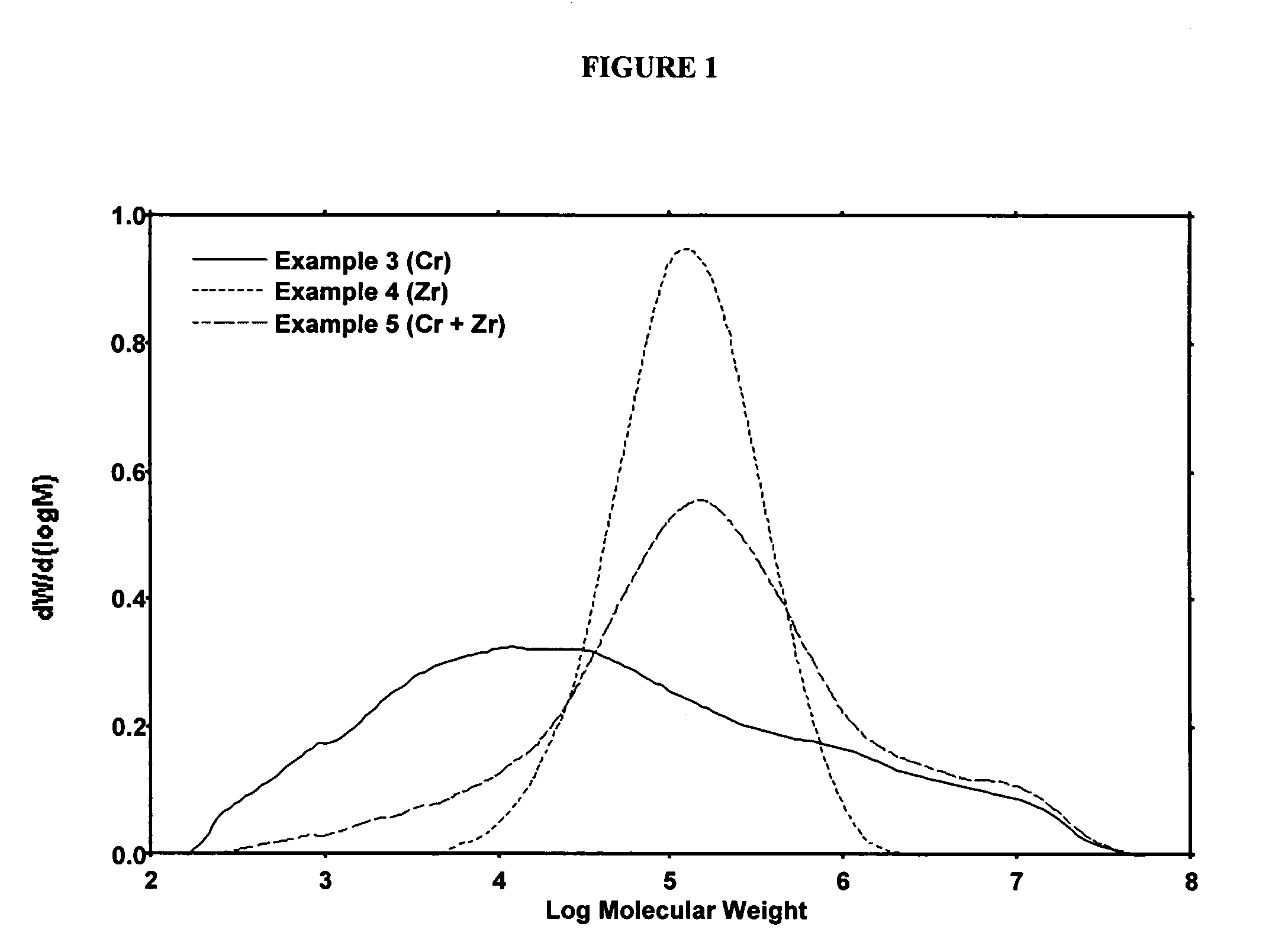
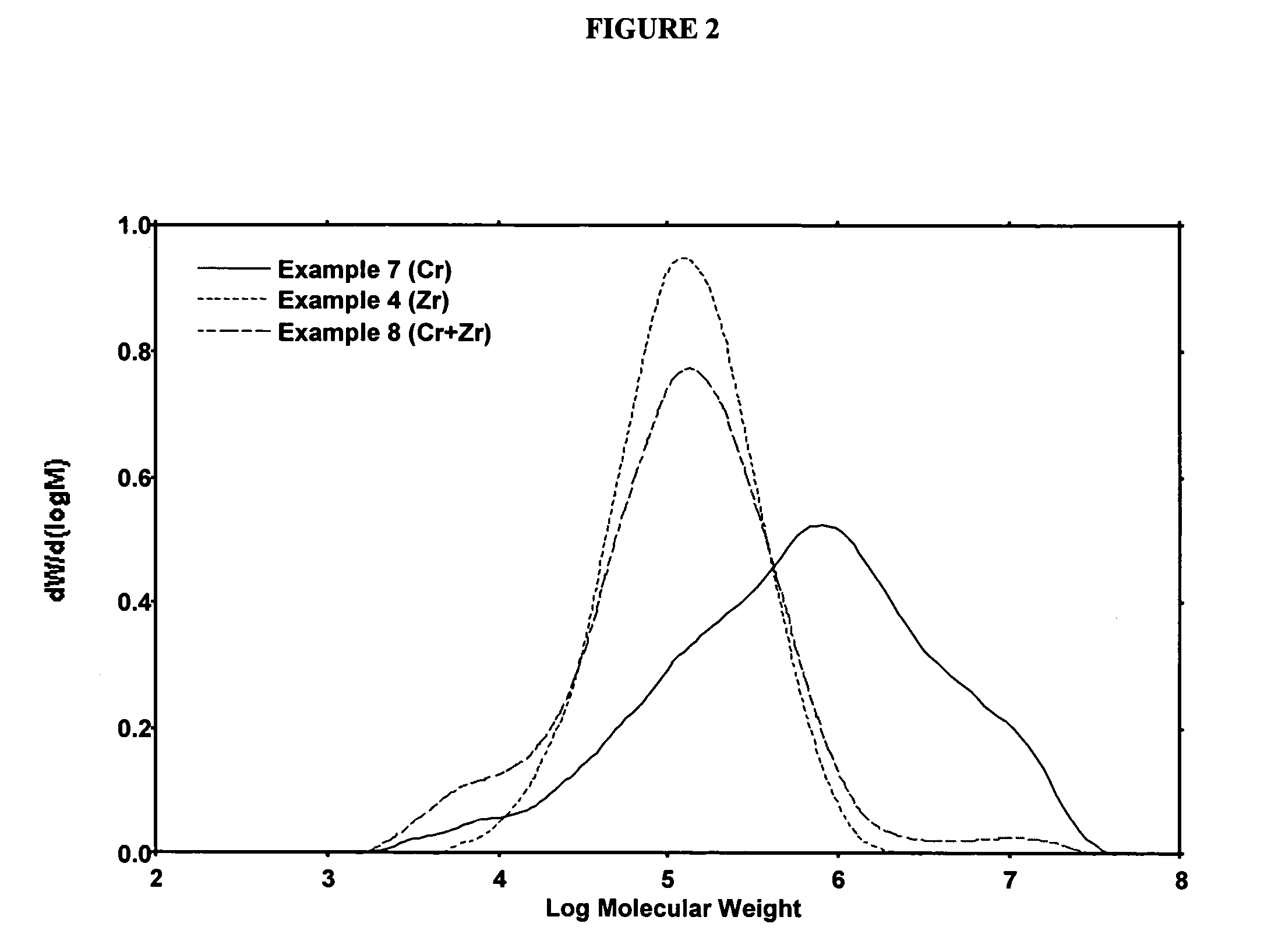
Polymerization Run Using the Dicumene Chromium Catalyst
[0246]To a dry 2.2 L steel reactor at about 90° C. was charged 0.5827 g of catalyst prepared as in Example 2. The reactor was equipped with a marine stirrer running at about 400 rpm and was surrounded by a steel jacket containing boiling methanol with a connection to a steel condenser. The boiling point of the methanol was controlled by varying nitrogen pressure applied to the condenser and the jacket, which permitted temperature control to within about 0.5° C. The run was allowed to proceed at an ethylene pressure of about 550 psig, a temperature of about 90° C., using isobutane as a diluent. As ethylene was consumed, more ethylene flowed in to maintain the pressure. The activity was noted by recording the flow of ethylene into the reactor to maintain the set pressure. After the allotted time, the ethylene flow was stopped and the reactor slowly depressurized and opened to recover a granular polymer powder. In all cases the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com