Well-defined donor-acceptor rod-coil diblock copolymer based on P3HT containing C60
a donor-acceptor rodcoil and copolymer technology, applied in the field of organic photovoltaic devices, can solve the problems of limited success of this approach, and achieve the effect of improving performance and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
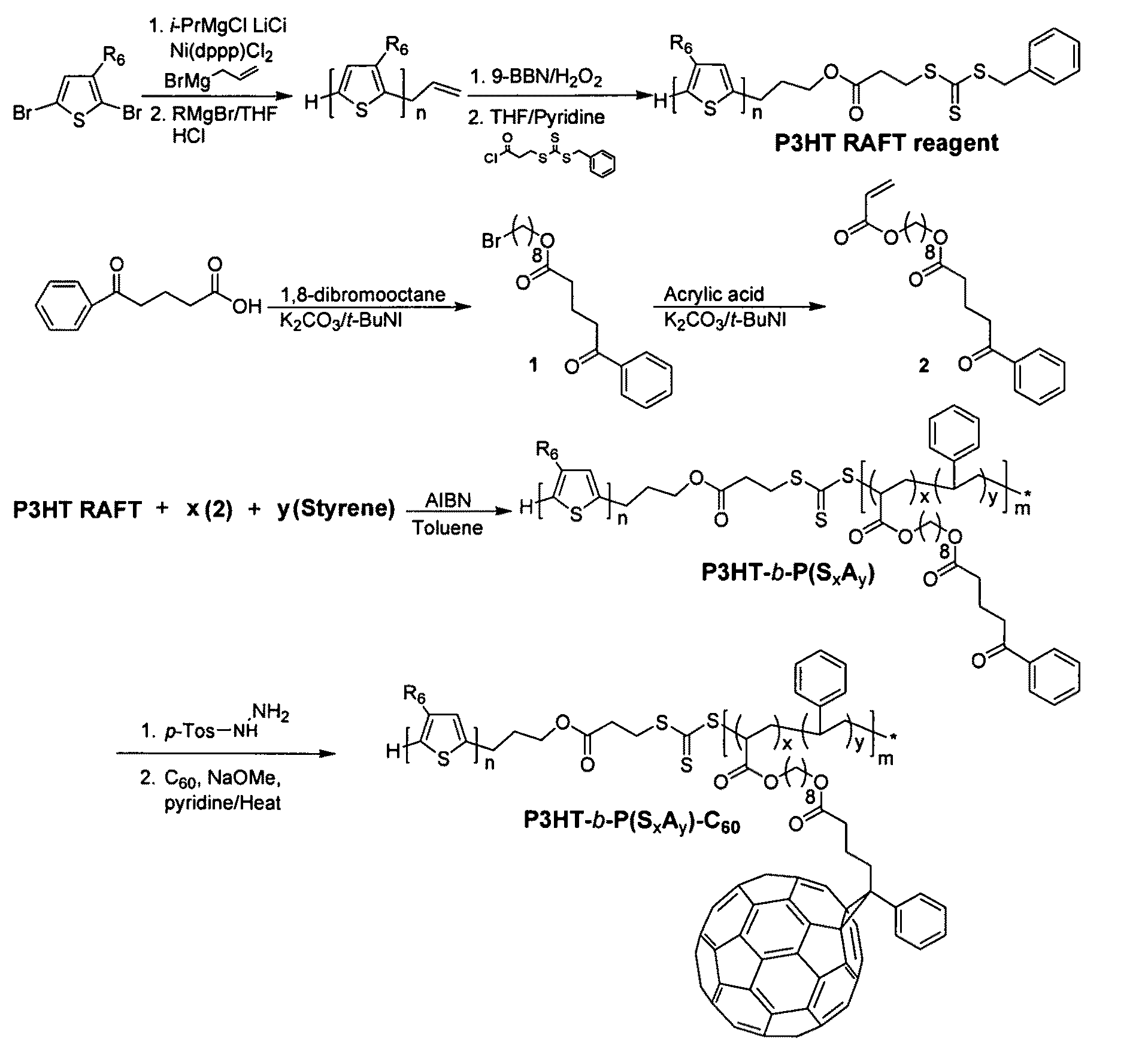
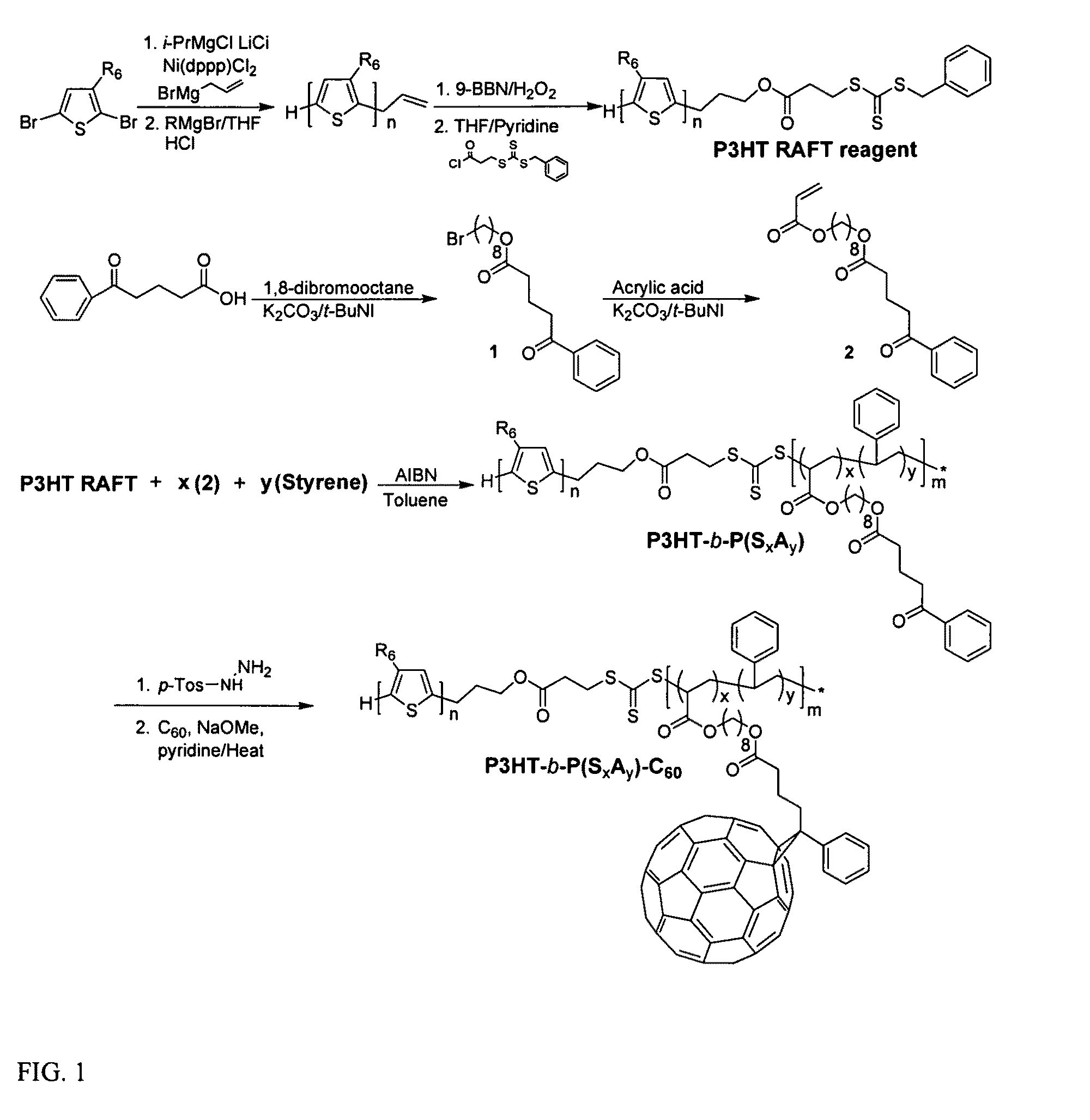
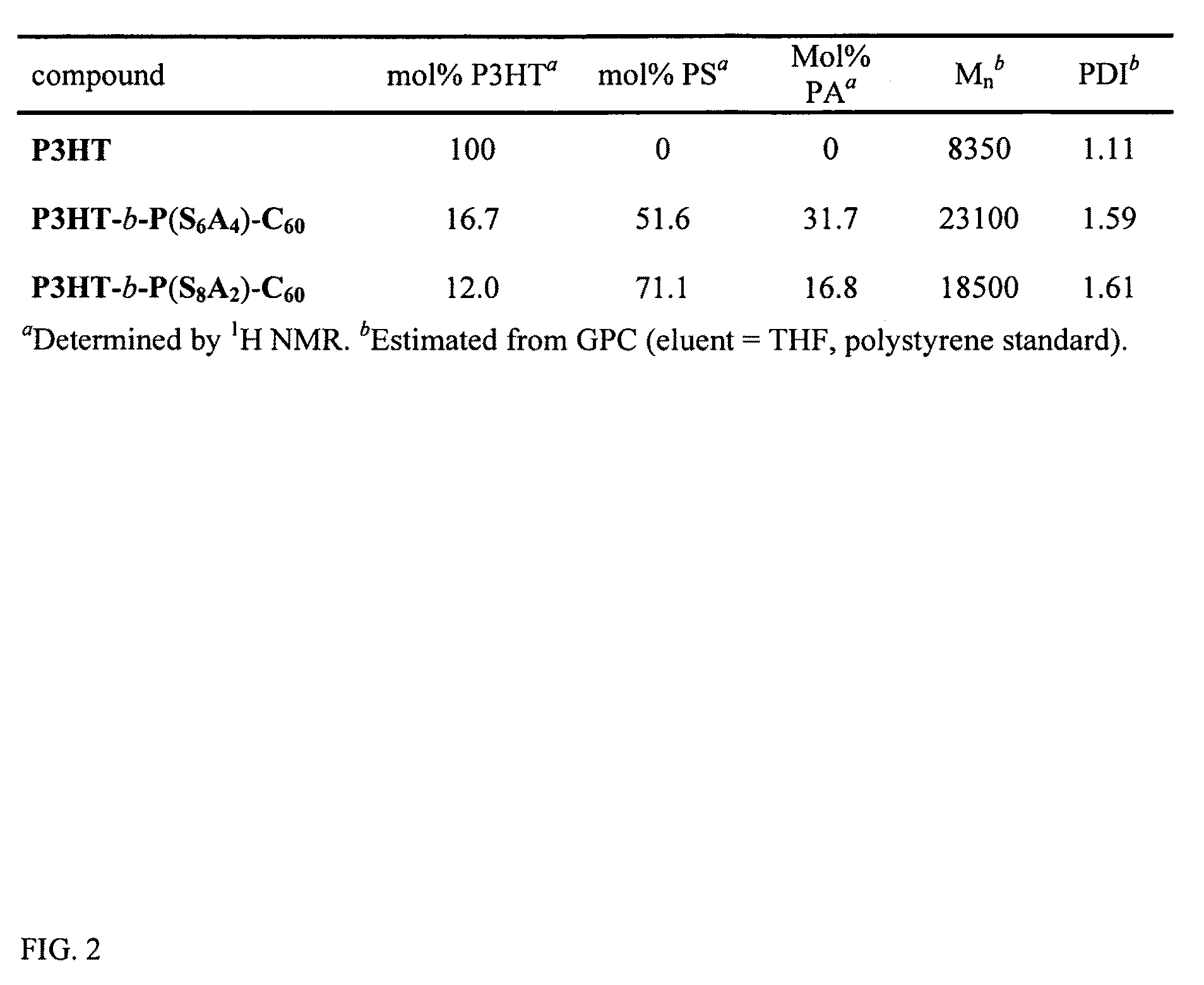
[0030]Among various living polymerization methods, reversible addition fragmentation chain transfer (RAFT) was selected for the synthesis of the rod-coil block copolymer containing polyolefin since it does not involve the use of a transition metal catalyst.33,34 This protocol is applicable to a whole range of monomers.35 The synthetic method is outlined in FIG. 1. The polymerization of 2,5-dibromo-3-hexylthiophene was performed by (GRIM) reaction with i-PrMgCl.LiCl / nickel catalyst36, followed by the treatment of a second Grignard reagent (allyl magnesium bromide), resulting in the mono end-capped P3HT (Mn=8.3 kg / mol, PDI=1.11, against PS standard). This is a slight synthetic modification of McCullough's method.25 The allyl-mono-capped P3HT typically contained two populations, one major and one minor, which correspond to allyl / Br and allyl / H respectively.37 To completely rule out the halogen defect on electronic applications, the allyl / Br fractions in the polymer were debrominated by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com