Chain transfer agent for addition mass polymerization of polycycloolefinic monomers
a polycycloolefinic monomer and chain transfer agent technology, applied in the field of chain transfer agent for addition mass polymerization of polycycloolefinic monomers, can solve the problems of high molecular weight polymers, inability to use in many applications involving solvent-based compositions, and large amount of solid and liquid waste, so as to reduce the amount of initiators, and control the effect of polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0250]The following abbreviations have been used hereinbefore and hereafter in describing some of the compounds, instruments and / or methods employed to illustrate certain of the embodiments of this invention:
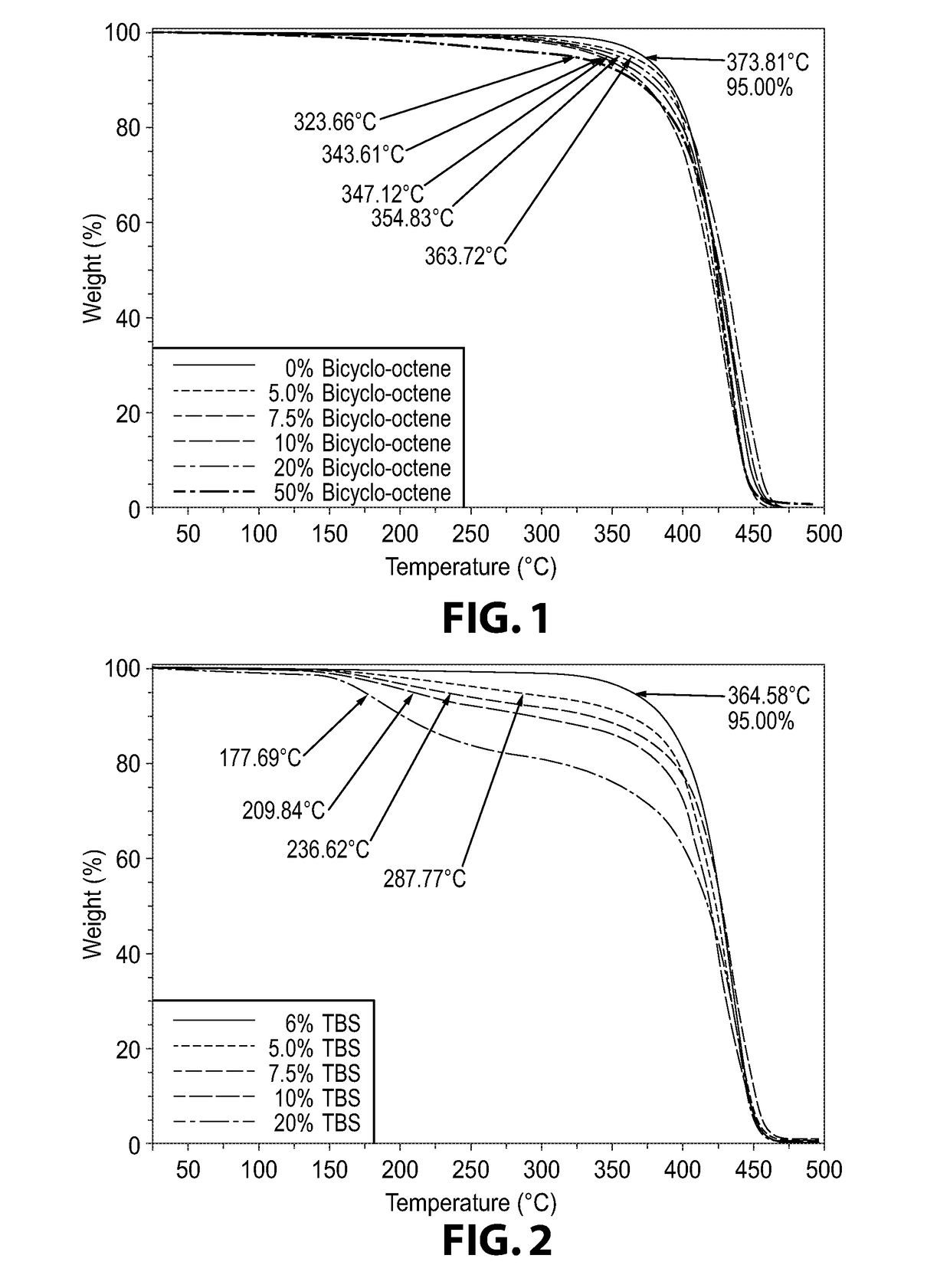
[0251]DecNB: 5-decylbicyclo[2.2.1]hept-2-ene; HexNB: 5-hexylbicyclo-[2.2.1]hept-2-ene; PENB: 5-phenethylbicyclo[2.2.1]hept-2-ene; BCO—bicyclo[4.2.0]oct-7-ene; DANFABA—N,N-dimethylaniliniumtetrakis(pentafluorophenyl)-borate; LiFABA—lithium tetrakis(pentafluoro-phenyl)borate; TBS—tri-n-butylsilane; THF—tetrahydrofuran; CTA—chain transfer agent; GPC: gel permeation chromatography; Mw—weight average molecular weight; PD—polydispersity; 1H NMR—proton nuclear magnetic resonance spectroscopy.
[0252]The following examples describe the procedures used for the preparation of various polymers as disclosed herein. However, it should be noted that these examples are intended to illustrate the disclosure without limiting the scope thereof.
[0253]The following Examples 1 to 11 illustrate the mas...
examples 1-5
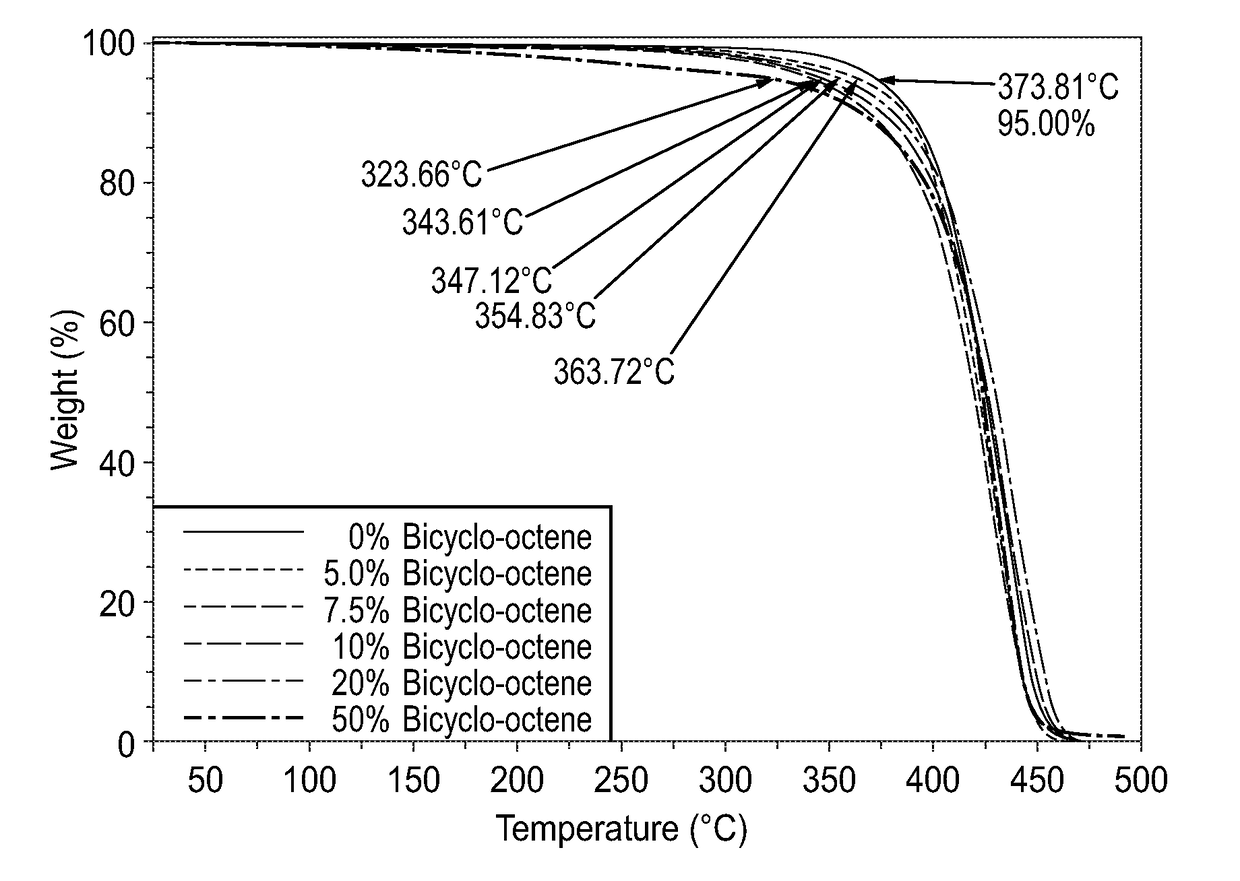
[0254]The following Examples 1-5 demonstrate the effects of various levels of BCO on homopolymer molecular weight.
[0255]Into a suitable reactor purged with nitrogen were placed 5000 parts of DecNB, one part of palladium compound (AcO—Pd—NCCH3(P-i-Pr3)2B(C6F5)4) and 2 parts of DANFABA. To this mixture was then added desirable amounts of BCO ranging from 1 mole percent to 7.5 mole percent based on the amount of monomer employed, as summarized in Table 1. The reaction mixture was then heated to 85° C. and maintained at this temperature for 30 minutes and then heated to 110° C. and maintained at that temperature for 30 minutes. After which time the reaction mixture was allowed to cool to room temperature. The resulting products in each of the Examples 1 to 5 were characterized by GPC in THF, the Mw and PD for each of the Examples 1 to 5 are summarized in Table 1. The thermal stability of each of the polymer products from Examples 1 to 5 were also analyzed by TGA, the temperature at whic...
examples 6-11
[0260]The procedures of Examples 1 to 5 were substantially repeated in these Examples 6 to 11 except that (iso-propoxy-dicyclopentadienyl)chloropalladium(triisopropyl)phosphine [Pd(i-PrO-DCPD)Cl(P-i-Pr3)] was used as the palladium initiator in combination with LiFABA in the ratio of 5000 parts of DecNB as the monomer, 1 part of palladium compound and 1 part of LiFABA. In addition, the mole percent of BCO employed were varied as summarized in Table 2. Also summarized Table 2 are the percent weight loss of the monomer, the Mw and PD as determined by GPC of each of the polymer products obtained and the 5% weight loss of the polymer product as determined by the TGA.
[0261]
TABLE 2Example Mol % % Weight GPCTGA, °C.No.BCOLossMw / PD(5% weight loss) 614.554,000 / 3.5369 73.34.836,000 / 3.5368 84529,000 / 3.4365 955.122,000 / 3354107.56.915,000 / 2.734611106.613,000 / 2.8345Comp. Ex. 207Insoluble363
[0262]It is again evident that BCO acts as an effective chain transfer agent in Examples 6-11. Furthermore, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com