Modified bacterial cellulose
a technology of bacterial cellulose and modified cellulose, which is applied in the direction of sugar derivates, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of not being able to produce modified bacterial cellulose, and achieve the effect of improving the modula of young peopl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0047]The culture medium used was composed of 50.0 g / l sucrose, 5.0 g / l “Total Amino Acid” (Ajinomoto Co., Inc.), 0.2 g / l phytic acid, 2.4 g / l magnesium sulfate and 1.0 g / l ammonium sulfate (pH 5.0).
[0048]Seed culture was carried out by placing 20 ml of the above culture medium in a 100 ml flask with baffle, inoculating Acetobacter pasteurianus FERM BP-4176, and then culturing at 25° C. for 3 days with stirring at 200 rpm. The culture medium was crushed by a blender, and added to a main culture medium having the above composition in a concentration of 2% seed culture.
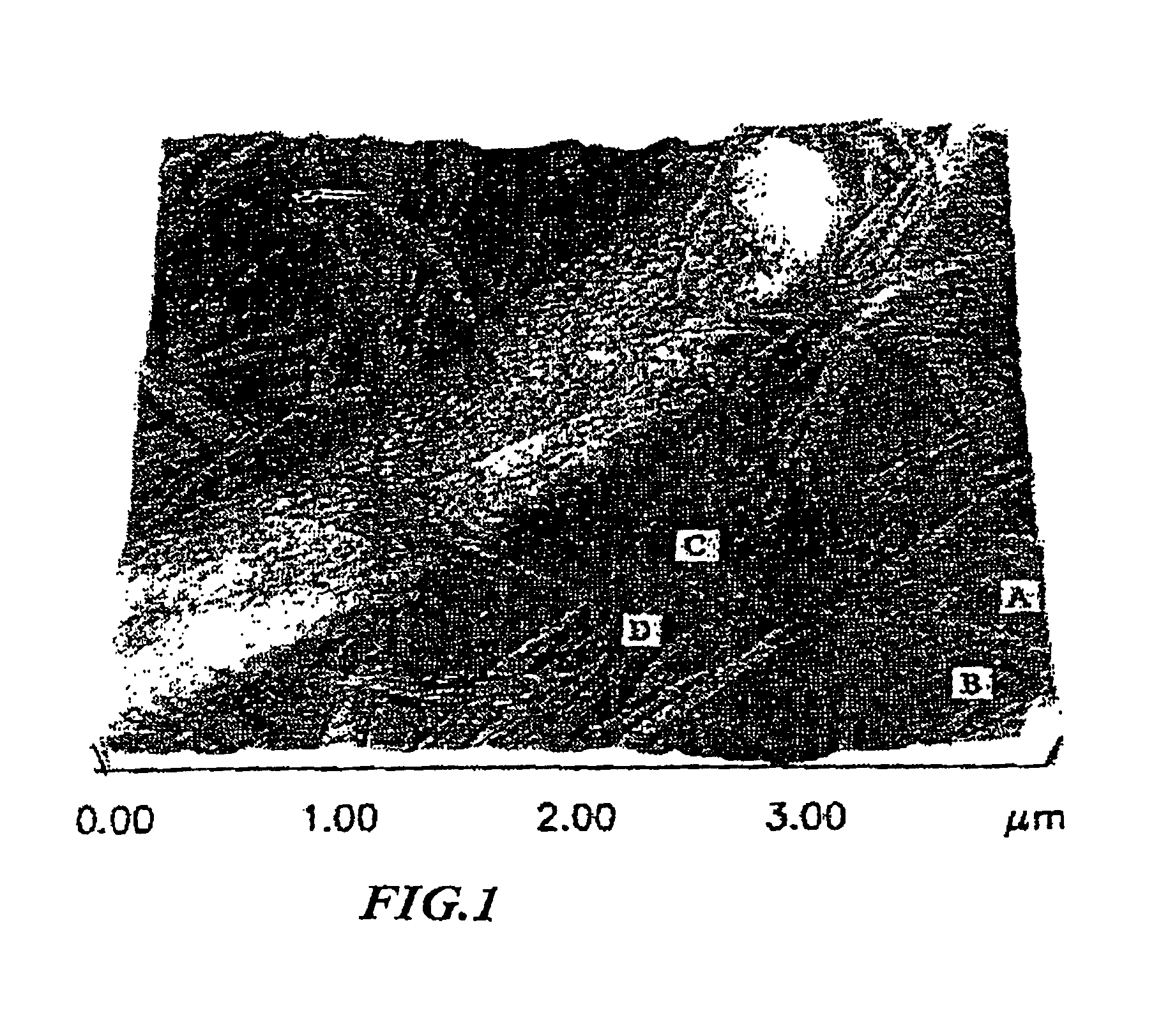
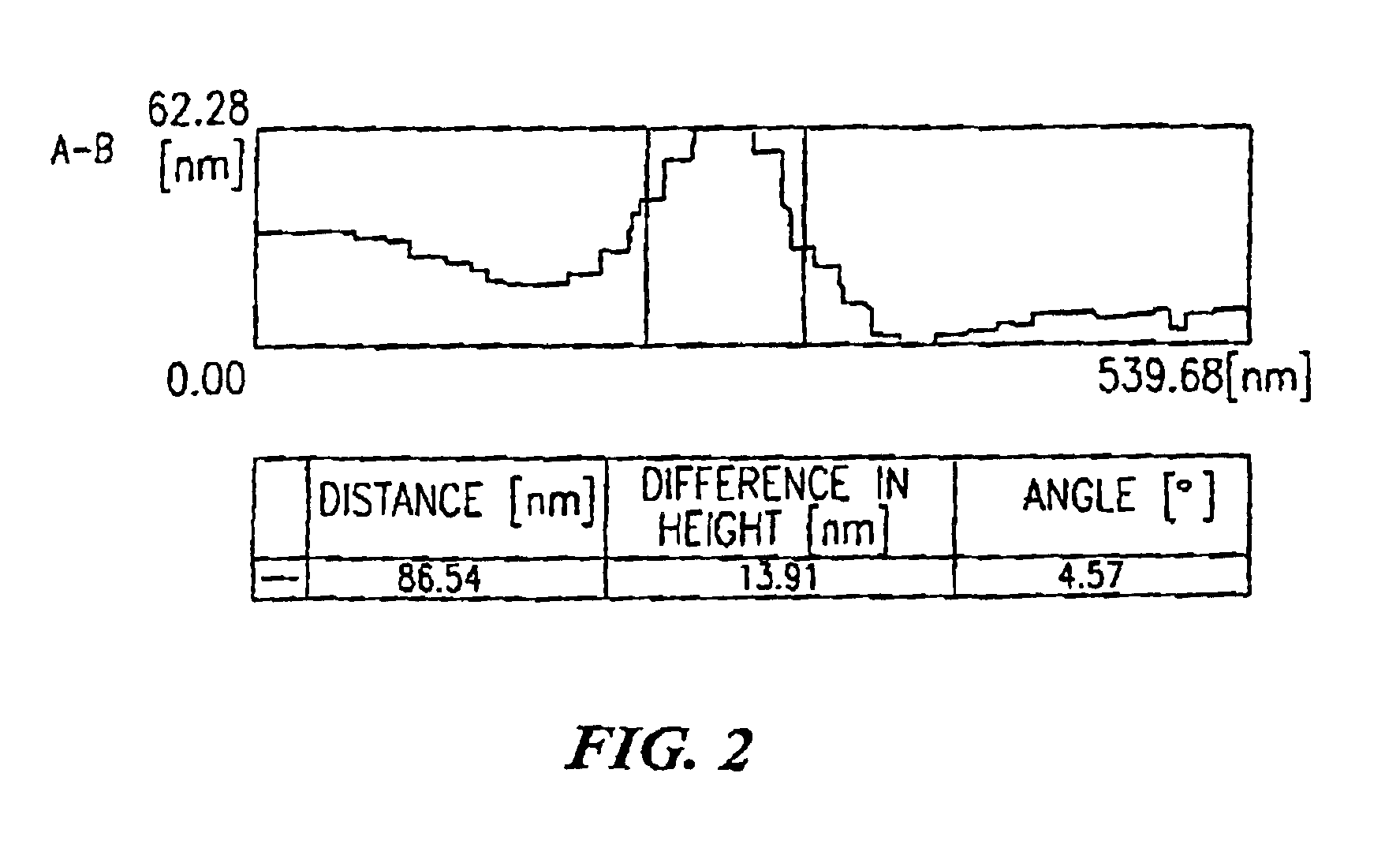
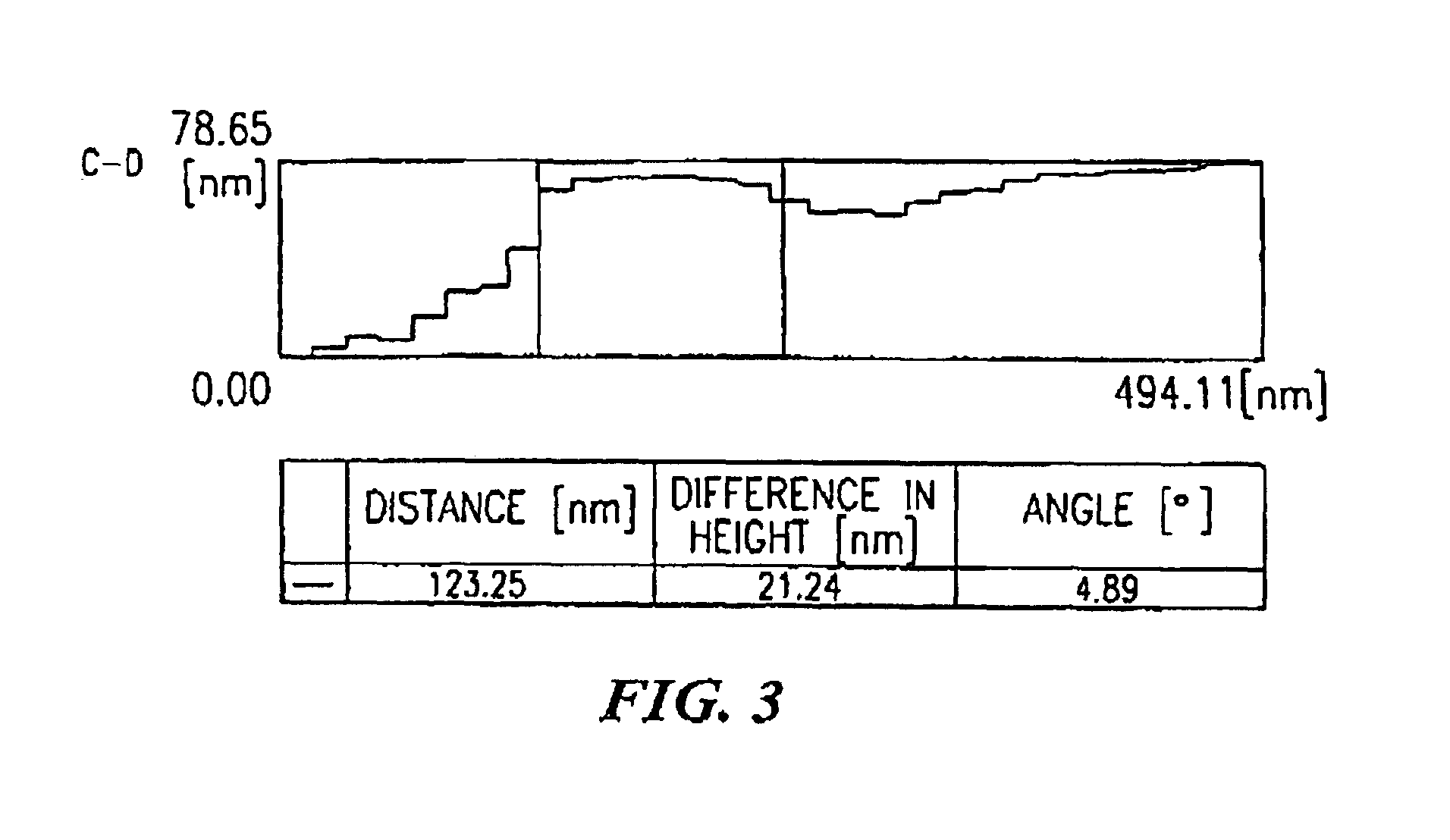
[0049]The main culture was carried out by static culture at 25° C. During the culture, culture solution and bacterial cellulose were withdrawn, and the morphology of bacteria was observed by an optical microscope, an electron microscope and an atomic force microscope.
[0050]Six main culture media were used, and nalidixic acid (NA) was added thereto in a concentration of 0.01 mM, 0.05 mM, 0.1 mM, 0.2 mM or 1.0 mM except o...
example 2
[0058]Acetobacter pasteurianus FERM BP-4176 was cultured in static culture, and the culture solution and bacterial cellulose were withdrawn, and the shape of bacteria was observed by the optical microscope, the electron microscope and the atomic force microscope, similar to Example 1, except that chloramphenicol was used instead of nalidixic acid.
[0059]That is, six main culture media having the aforementioned composition were used, and chloramphenicol (CP) was added thereto in a concentration of 0.1 mM, 0.2 mM, 0.3 mM, 0.5 mM or 1.0 mM except one medium to which CP was not added.
[0060]As a result, the length of the cellulose-producing bacterium increased with increasing the CP concentration up to 8 to 12 times as long as the bacteria cultured in no CP medium.
[0061]As an example, the shape of bacterium cultured in the 0.3 mM CP medium for 2 days taken by the optical microscope (×1000), and shown in FIG. 4, and that cultured in no CP medium for 2 days is shown in FIG. 5.
[0062]The CP r...
example 3
[0066]Acetobacter pasteurianus FERM BP-4176 was cultured in static culture, and the culture solution and bacterial cellulose were withdrawn, and the form of bacteria was observed by the optical microscope, the electron microscope and the atomic force microscope, similar to Example 1, except that chloramphenicol was used instead of nalidixic acid.
[0067]That is, four main culture media having the aforementioned composition were used, and dithiothreitol (DTT) was added thereto in a concentration of 0.5 mM, 1.0 mM or 2.0 mM except one medium to which DTT was not added.
[0068]As a result, the length of the cellulose-producing bacterium decreased with increasing the DTT concentration.
[0069]As an example, the shape of bacterium cultured in the 1.0 mM DTT medium for 2 days taken by the optical microscope, and shown in FIG. 7. As can be seen from the photograph, the length of the bacterium cultured in 1.0 mM DTT medium wat shortened to ⅓ to ½ of the bacteria cultured in no DTT medium.
[0070]Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com