Methods of treating fibrosis, cancer and vascular injuries
a fibrosis and cancer technology, applied in the field of fibrosis, cancer and vascular injuries, can solve problems such as complicated organ transplantation, and achieve the effects of reducing the number of patients, preventing and treating various disease states, and facilitating the transplantation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
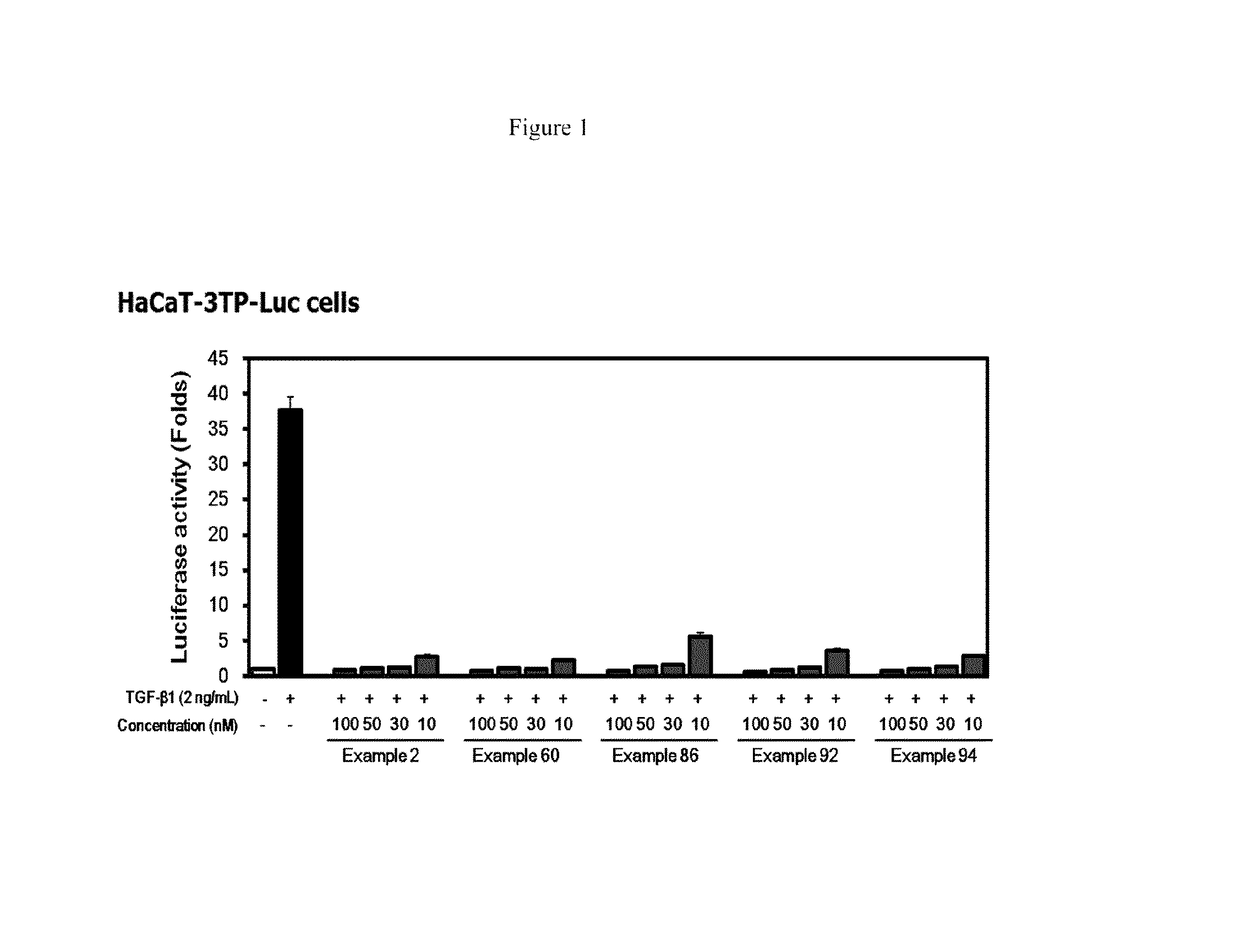
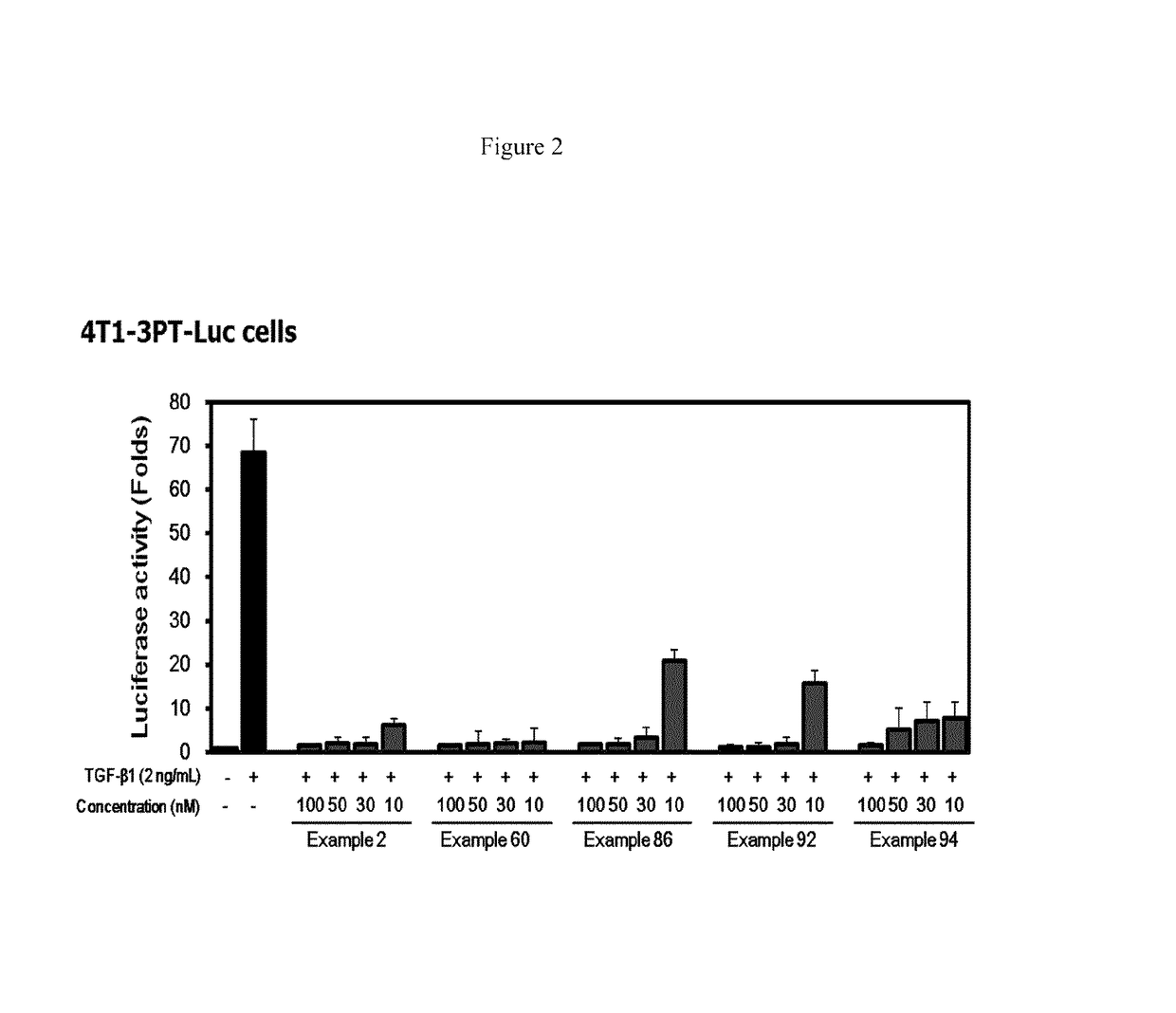
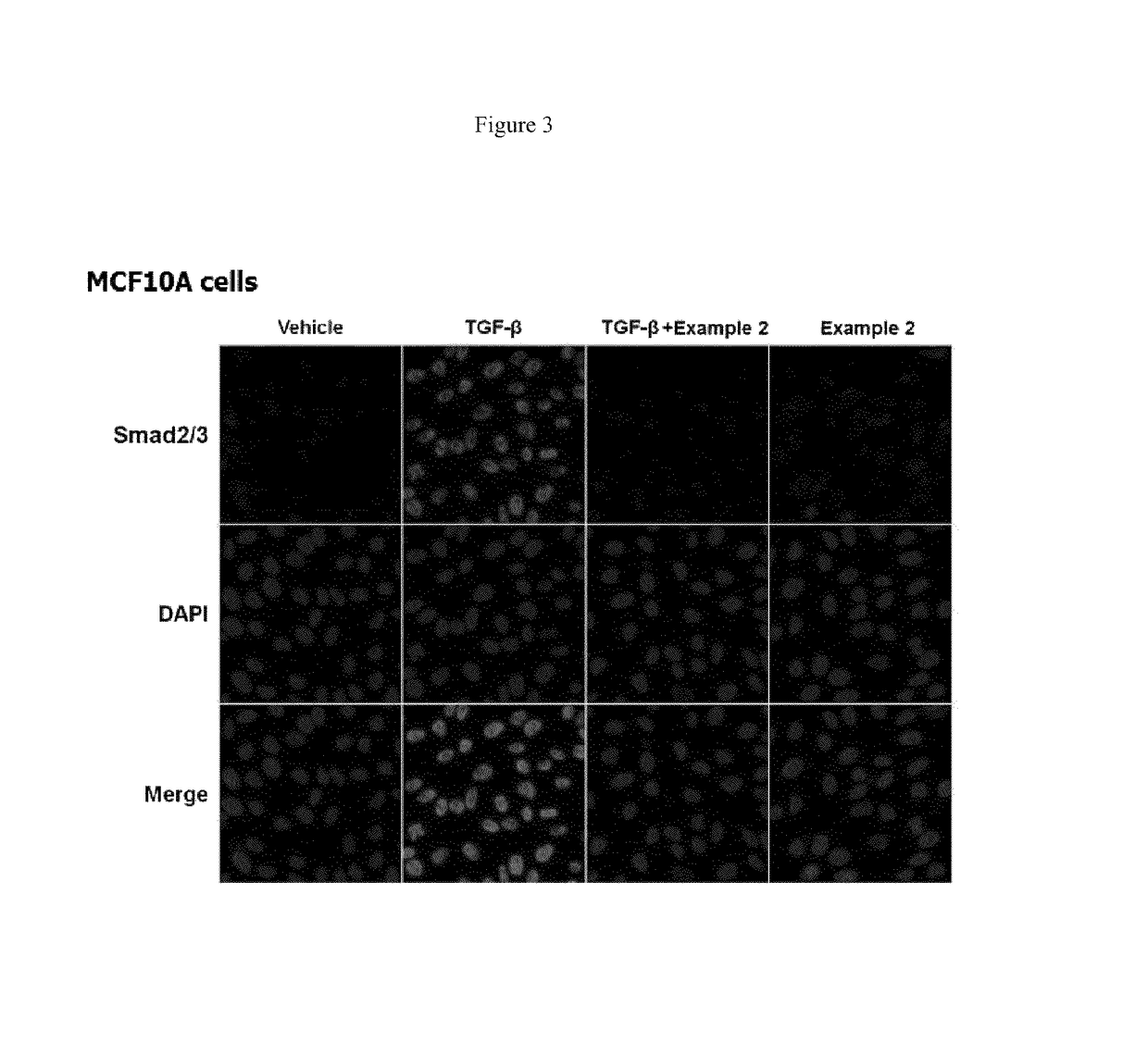
[0215 suppressed TGF-β1-induced cell migration in MCF10A cells.
[0217]The upper surface of Transwells (6.5 mm diameter, 8 μm pore size; Corning, Lowell, Mass., USA) were coated with 20 μL diluted Matrigel (BD Biosciences). 4T1 cells were seeded at 4×104 cells / well on the upper chamber of transwell in serum free medium with or without TGF-β1 (2 ng / mL) in the presence or absence of Example 2. The lower chamber was filled with 10% FBS with TGF-β1 (2 ng / mL) in the presence or absence of Example 2. After incubation for 20 h at 37° C. in 5% CO2, the cells remaining on the upper surface of the membrane were removed with a cotton swab, and DAPI-stained cells remaining on the bottom surface were observed using fluorescence microscopy. Average cell number per view field was obtained from 5 random fields.
[0218]Example 2 suppressed TGF-β1-induced cell invasion in matrigel invasion assay.
[0219]Cell Growth Study
[0220]Either 4T1 cells or MCF10A cells were seeded in 96-w...
example 61
[0228 significantly reduced the number of metastastic lesions in the lung. Significant level of β-casein (a mammary differentiation marker) mRNA was detected in the lung of MMTV / c-Neu mice. Examples 3 and 61 significantly inhibited β-casein mRNA expression level in the lung, demonstrating their anti-metastatic effect.
[0229]Activity of MMP-9 and MMP-2 in the primary mammary tumor was measured by gelatin zymography. Tumor tissues from mice (30 mg) was lysed in 500 μL RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1% NP-40, protease inhibitor without EDTA) for 10-20 min on ice. Lysates were cleared by centrifugation at 13000 rpm at 4° C. for 10 min. Protein content of supernatants was determined using Micro-BCA protein assay kit (Thermo Scientific). Loading samples were prepared by adding loading buffer (0.5 M Tris, pH 6.8, 50% glycerol, 10% SDS, and 1% bromophenol blue solution) into lysates containing 15 μg of total protein. Loading sampl...
example 3
[0230 significantly inhibited activity of MMP-9 and MMP-2 in the primary mammary tumor.
[0231]Anti-Fibrotic Effect on Bile Duct-Ligated Liver Fibrosis Model
[0232]Six-week-old male Sprague-Dawley (SD) rats were purchased from Orient Bio Inc. In Experiment 1, SD rats weighing 180-200 g were randomly divided into five experimental groups: sham-operated control rats (n=5), sham-operated rats treated with Example 3 (43.6 mg / kg, n=5), bile duct-ligated (BDL) rats (n=10), BDL rats treated with either 21.8 or 43.6 mg / kg of Example 3 (n=10). In Experiment 2, SD rats weighing 180-200 g were randomly divided into five experimental groups: sham-operated control rats (n=5), BDL rats (n=10), BDL rats treated with either 5, 10, or 20 mg / kg of Example 2 (n=10). For BDL, the animals were anesthetized with zoletil (20 mg / kg) and xylazine (10 mg / kg), and the common bile duct was exposed and double-ligated using 3-0 silk. The first ligature was placed below the junction of the hepatic duct, and the seco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com