Drug delivery device for providing local analgesia, local anesthesia or nerve blockage
a delivery device and local anesthesia technology, applied in the direction of prosthesis, heterocyclic compound active ingredients, amide active ingredients, etc., can solve the problems of increasing the possibility of post-surgical complications, increasing the cost of medical care, and affecting recovery and recovery. the effect of normal activities of daily living
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
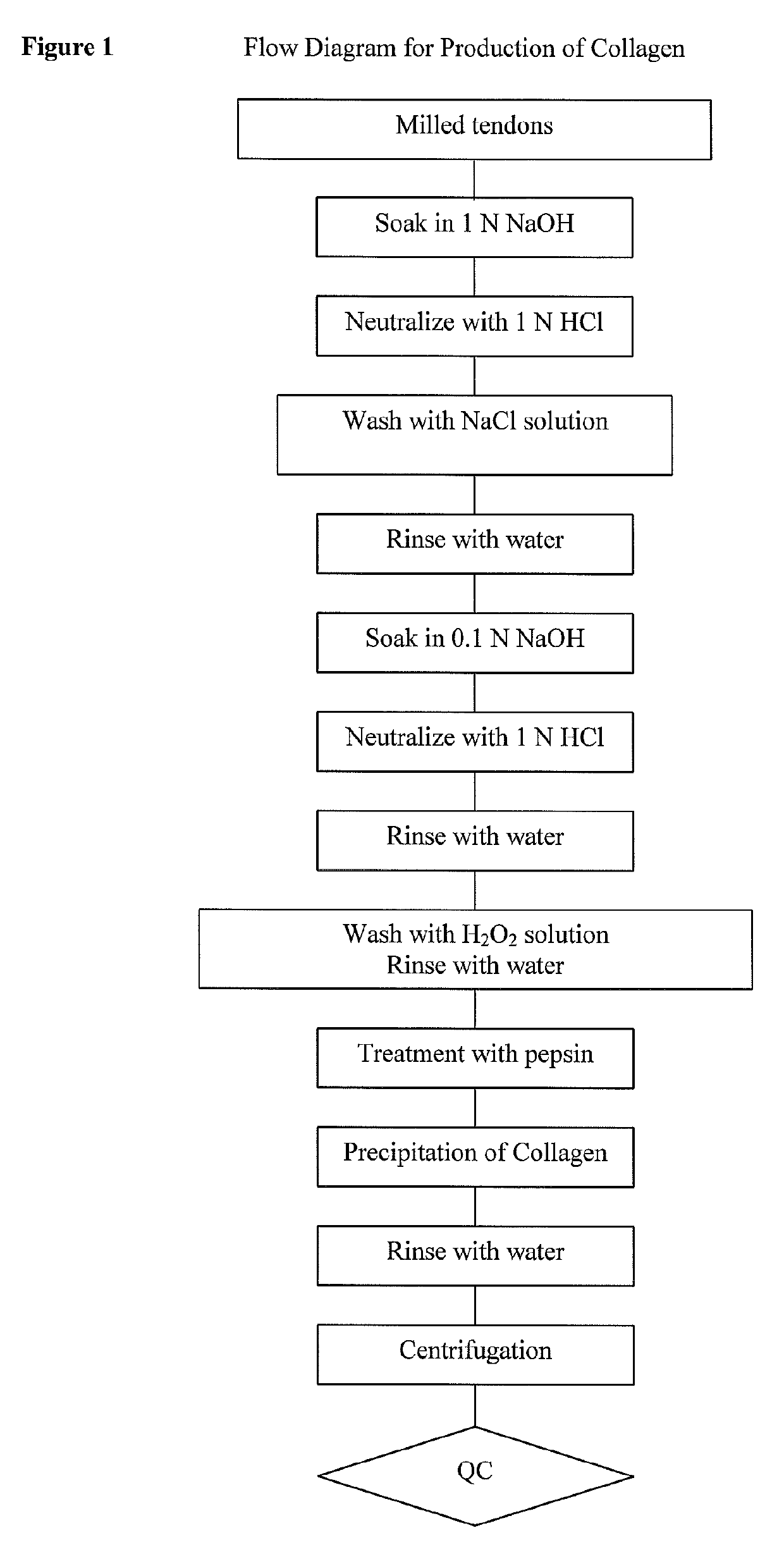
[0076]The effective relief of pain is of paramount importance to those treating patients undergoing surgery. This should be achieved for humanitarian reasons, but there is now evidence that pain relief has significant physiological benefit. Not only does effective pain relief mean a smoother postoperative course with earlier discharge from hospital, but it may also reduce the onset of chronic pain syndromes. Topical or local administration of anesthetics directly at the surgical site has the advantage of producing high local anesthetic concentrations, while minimizing potentially toxic systemic concentrations.
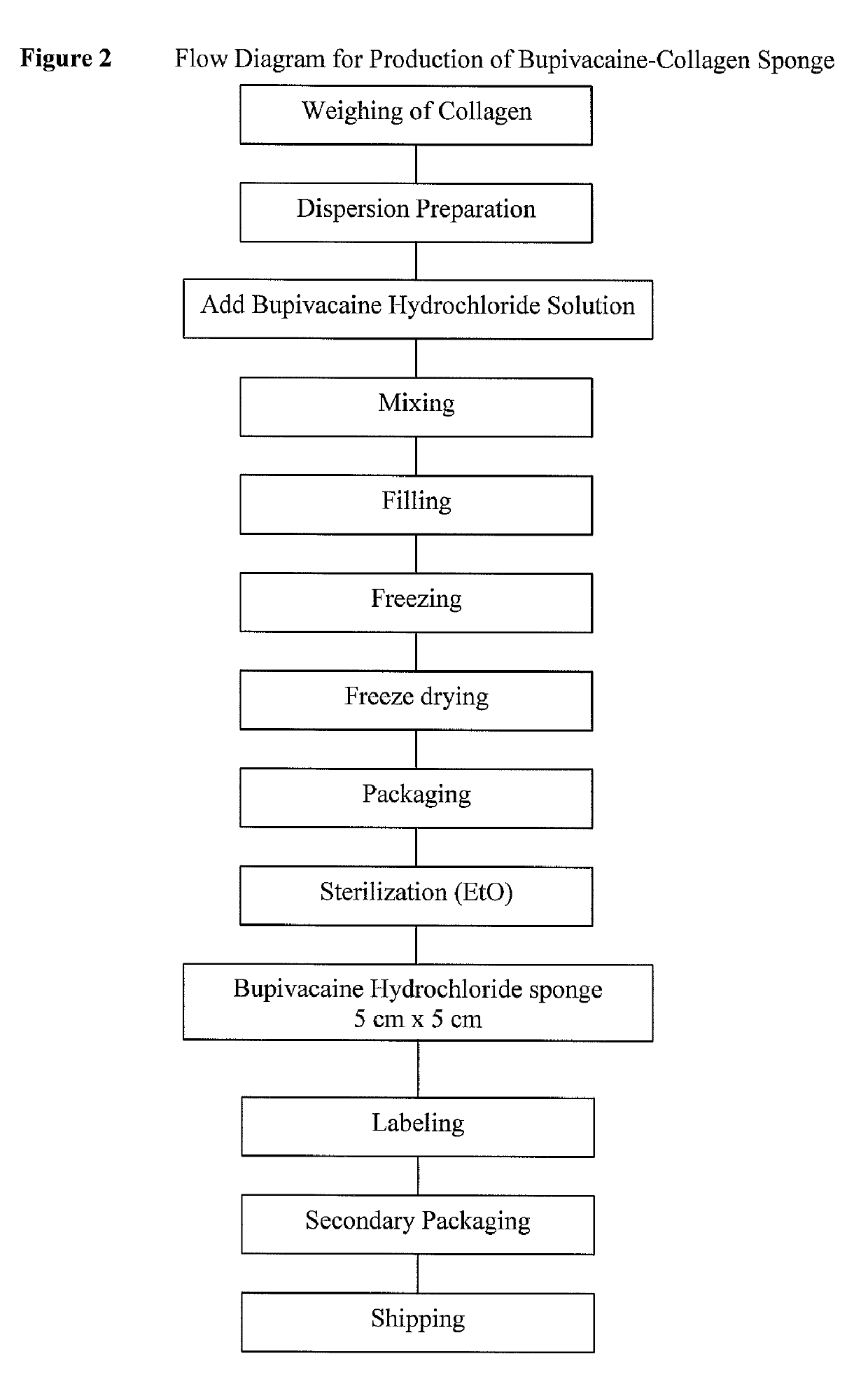
[0077]The bupivacaine-collagen sponge is highly malleable and can be applied directly, rolled or folded, giving the surgeon great flexibility in terms of application in wounds scheduled for closure.
[0078]Patients received three 5 cm×5 cm (×0.5 cm thick) sponges; one sponge divided between areas adjacent the surgical site (in this case, adjacent the location of the...
example
Clinical Study-Dose Determination for Abdominal Hysterectomies and Other Non-Laparoscopic Benign Gynecological Procedures Such as Myomectomy and Adnexal Surgery
[0129]The drug delivery device of the present invention is designed to provide prolonged, local analgesia by direct application of the drug delivery device to the site of tissue disruption. However, it should be emphasized that the drug delivery device of the present invention is not expected to provide complete relief from all postoperative pain or entirely eliminate the need for rescue analgesia medication but, instead, is intended as part of multimodal therapy for safe and effective pain management.
[0130]Each bupivacaine-containing drug delivery sponge has a surface area of 25 cm2 (5×5 cm) and a thickness of approximately 0.5 cm. The active ingredient (bupivacaine hydrochloride) is homogenously dispersed throughout the collagen drug delivery matrix and, on a surface area basis, has a concentration of 2 mg / cm2. This concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com