Charge reversal Pulullan derivative and synthesis method and application thereof
A technology of pullulan polysaccharide and charge reversal, which is applied in the field of charge reversal pullulan polysaccharide derivatives and their synthesis, which can solve the problems of in vivo application limitations, lack of tumor targeting, etc., and achieve simple synthesis process and no immunogenicity , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
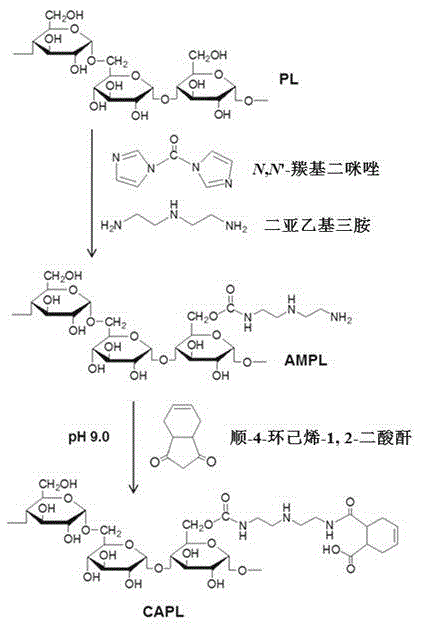
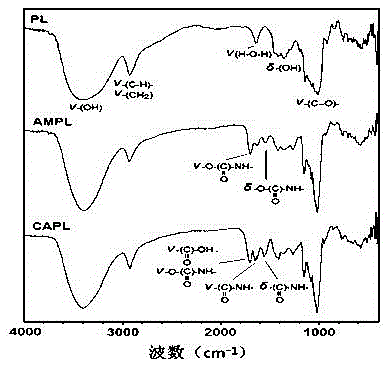
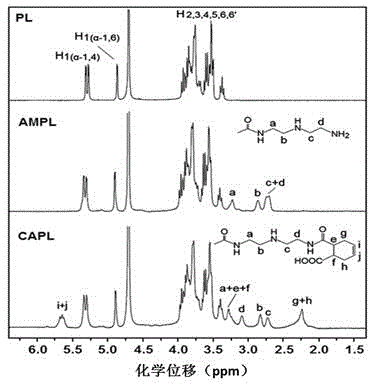
[0048] 1. Synthesis of diethylenetriamine-modified pullulan (AMPL) (e.g. figure 1 )
[0049] Dissolve 500mg of pullulan (molecular weight 200kDa) and 500mg of N,N'-carbonyldiimidazole (CDI) in 20ml of anhydrous dimethylsulfoxide (DMSO) and stir for 5-10min, then slowly at a speed of 1-2ml / min Drop into 10ml of DMSO solution dissolved with 2ml of diethylenetriamine, and stir at 35°C for 20h. After the reaction, the reaction solution was transferred to a dialysis bag (molecular weight cut-off 12-14kDa), dialyzed in ultrapure water for 3 days, and the dialysate was freeze-dried for 40 hours. The white flocculent product obtained was AMPL. The degree of substitution of the polyamino-modified group can be calculated by detecting the content of nitrogen element by means of elemental analysis, which is 15.7%.
[0050] 2. Synthesis of cis-4-cyclohexene-1,2-dicarboxylic acid monoamidation-diethylenetriamine-modified pullulan (CAPL) (e.g. figure 1 ).
[0051] Dissolve 200mg of the...
Embodiment 2
[0075] 1. Synthesis of spermine-modified pullulan (SPL)
[0076] Dissolve 500mg of pullulan (molecular weight 200kDa) and 500mg of N,N'-carbonyldiimidazole (CDI) in 20ml of anhydrous dimethylsulfoxide (DMSO) and stir for 5-10min, then slowly at a speed of 1-2ml / min Drop into 10ml of DMSO solution dissolved with 5ml of spermine, and stir at 35°C for 24h. After the reaction, the reaction solution was transferred to a dialysis bag (molecular weight cut-off 12-14kDa), dialyzed in ultrapure water for 3 days, and the dialysate was freeze-dried for 40 hours. The obtained white flocculent product was SPL. The degree of substitution of the spermine-modified group was 37.9%.
[0077] 2. Synthesis of maleic acid monoacylation-spermine modified pullulan (MSPL)
[0078] Dissolve 200 mg of the above SPL in 10 ml of DMSO, add 100 mg of maleic anhydride and stir for 24 hours, then pour the reaction solution into cold ethanol solution, a white flocculent precipitate is precipitated, collec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com