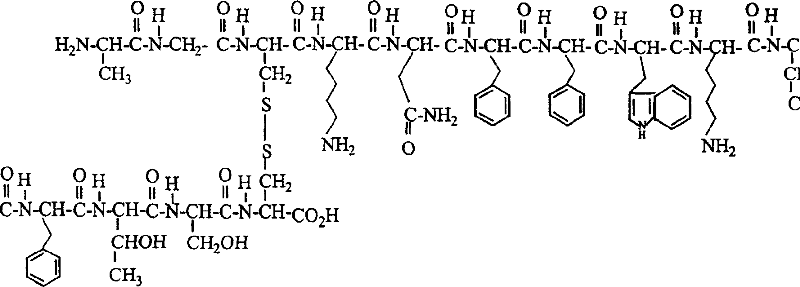
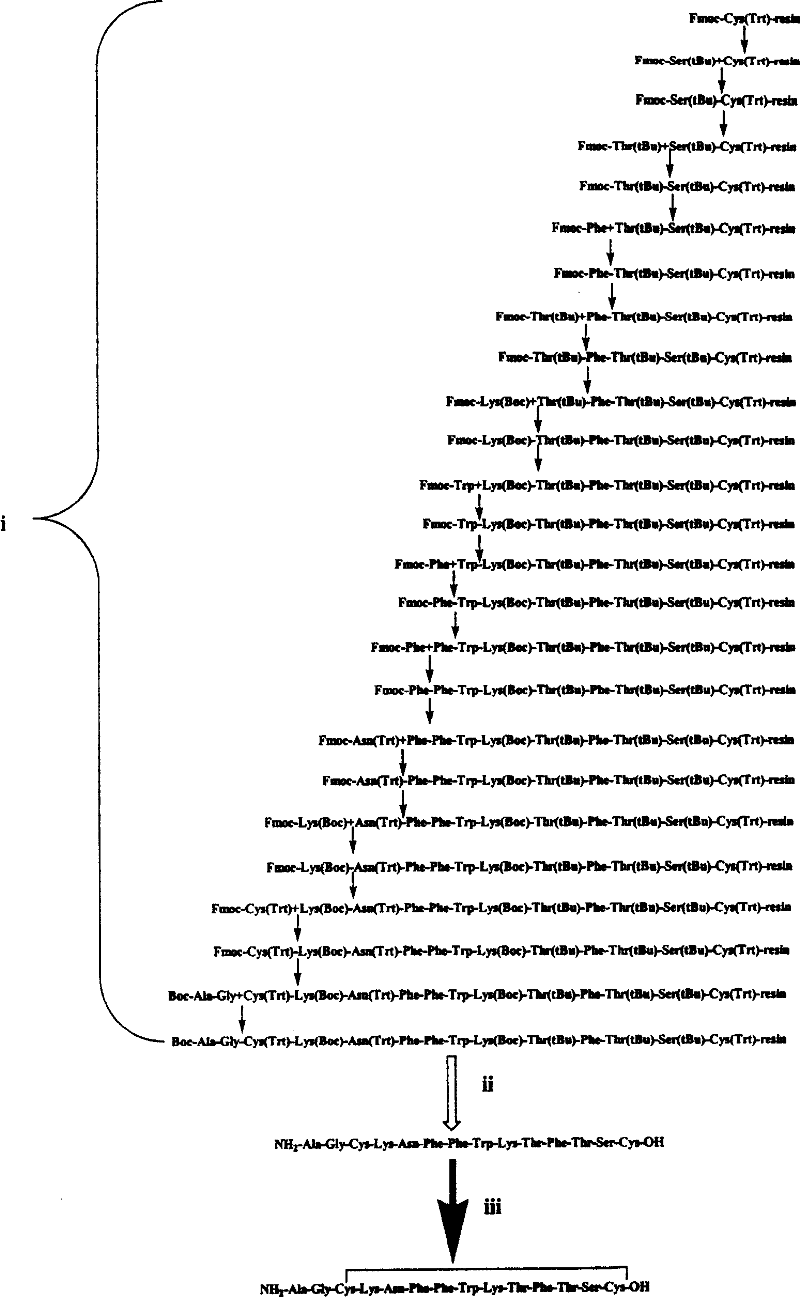
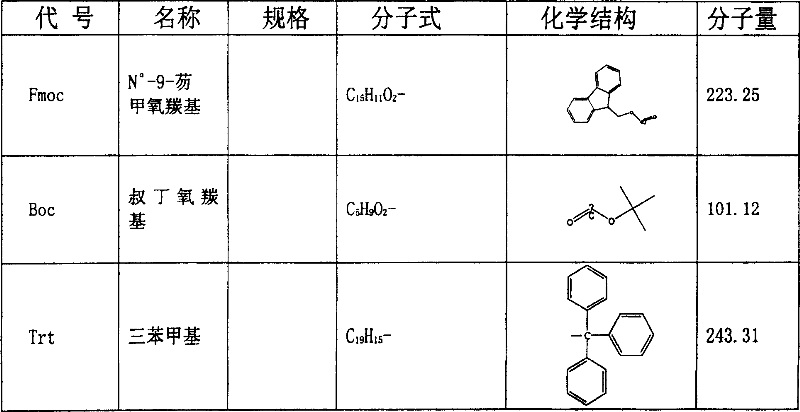
Somatostatin full-synthesis method
A somatostatin, total synthesis technology, applied in the biological field, can solve the problems of many reaction steps, lengthy routes, and difficulty in solvent distillation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]N α -9-fluorenylmethoxycarbonyl-O-tert-butyl-seryl-N min - Preparation of trityl-cysteinyl-2-chloro-trityl resin
[0050]
[0051]
[0052] Reagent
Feeding amount
moles
N α -9-fluorenylmethoxycarbonyl
-O-tert-butyl-serine
Fmoc-Ser(tBu)-OH
1.54g
4mmol
Fmoc-Cys(Trt)-(R)
4g
2mmol
HOB
0.54g
4mmol
DCC / DCM solution
4ml
4mmol
20% piperidine / DMF solution
50ml×2
DCM
about 320ml
MeOH
about 300ml
DMF
Appropriate amount
[0053] Operation: (1) Put the Fmoc-Cys(Trt)-(R) resin in a peptide synthesizer, alternately wash twice with DCM and MeOH each 30ml×2, 2min / time, drain, add 20% piperidine / DMF solution 50ml×2 room temperature. Stir for 20 min, 2 times (to remove Fmoc), drain, and wash the resin alternately with DCM and MeOH each 30ml×3 for 3 times, 2 min each time, and drain. Kasier reagent test, the resul...
Embodiment 2
[0060] N α -9-fluorenylmethoxycarbonyl-O-tert-butyl-threonyl-O-tert-butyl-seryl-N im - Preparation of trityl-cysteinyl-2-chloro-trityl resin
[0061] Reaction formula:
[0062]
[0063]
[0064] Reagent
Feeding amount
moles
resin
2mmol
Fmoc-Thr(tBu)
-OH
1.6g
4mmol
HOB
0.54g
4mmol
DCC / DCM solution
4ml
4mmol
20% piperidine / DMF solution
liquid
100ml
DCM
about 320ml
MeOH
about 300ml
DMF
Appropriate amount
[0065] Operation: (1) Add 20% piperidine / DMF solution 50ml×2 to the resin, stir at room temperature for 20min, twice (to remove Fmoc), drain, and wash the resin alternately with 30ml×3 DCM and MeOH for 3 times, 2min / Once, drain the Kaiser reagent. Test, the result is positive, Fmoc has been removed, if it is negative, continue to go to Fmoc until it is positive.
[0066] (2) Add the DCM / DMF solution containi...
Embodiment 3
[0072] N α -9-fluorenylmethoxycarbonyl-phenylalanyl-O-tert-butyl-threonyl-O-tert-butyl-seryl-N im - Preparation of trityl-cysteinyl-2-chloro-trityl resin
[0073] Reaction formula:
[0074]
[0075]
[0076]
[0077] Reagent
Feeding amount
moles
resin
2mmol
Fmoc-Phe-OH
1.55g
4mmol
HOB
0.54g
4mmol
DCC / DCM solution
4ml
4mmol
20% piperidine / DMF solution
100ml
DCM
about 320ml
MeOH
about 300ml
DMF
Appropriate amount
[0078] Operation: (1) Add 20% piperidine / DMF solution 50ml×2 to the resin, stir at room temperature for 20min, twice (to remove Fmoc), drain, and wash the resin alternately with 30ml×3 DCM and MeOH for 3 times, 2min / Once, drain the Kaiser reagent. Test: The result was positive, Fmoc has gone.
[0079] (2) Add the DCM / DMF solution containing Fmoc-Phe-OH and HOBt to the resin, add 4ml of DCC / DCM solution, and stir a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com