Bone marrow interstital stem cell preparation and its combined use with controlled release neurotrophic factor
A technology of central nervous system and composition, which is applied in the direction of drug combination, nervous system diseases, microorganisms, etc., to achieve great reference value, convenient application, and reliable experimental results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
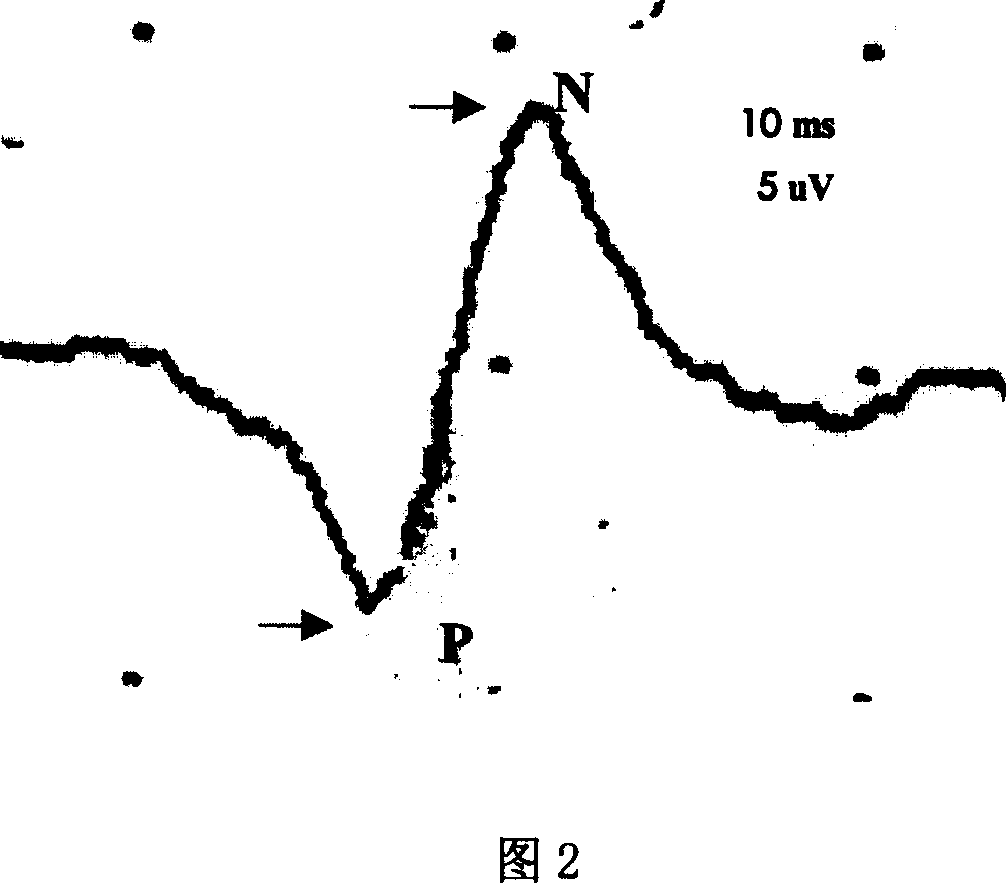
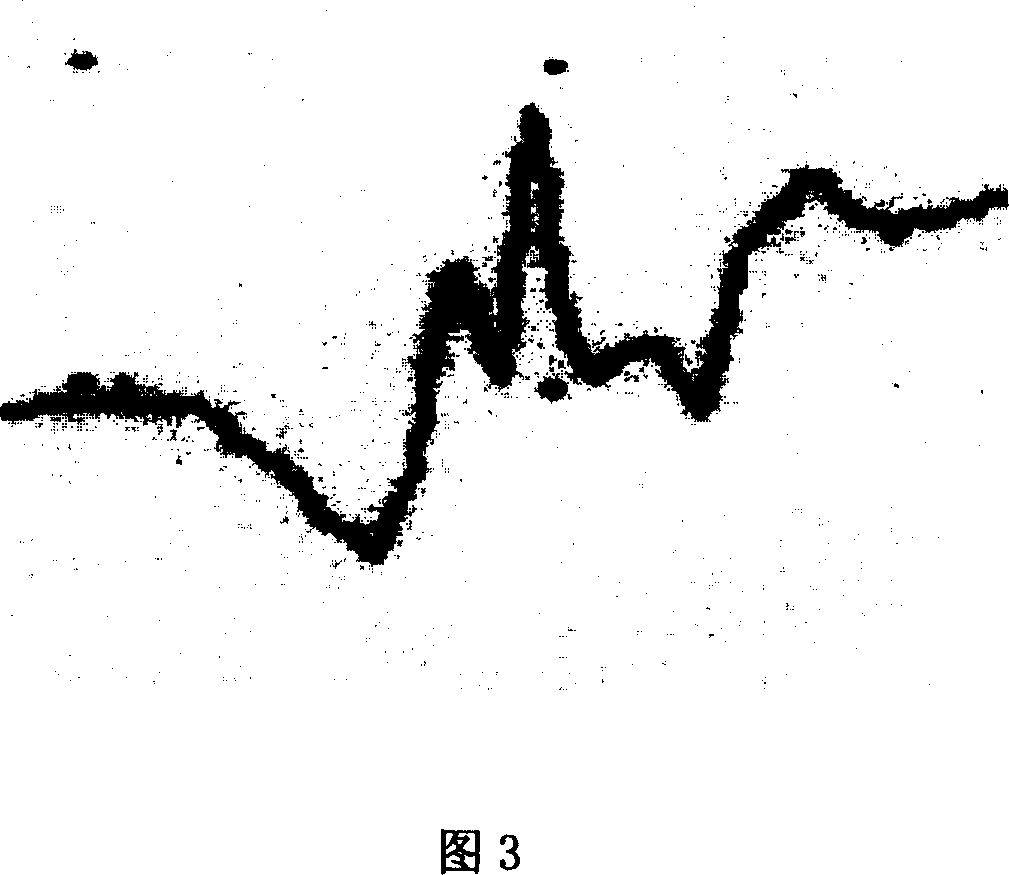
Image
Examples
Embodiment 1
[0033] Example 1. Example of preparation technology of improved bone marrow mesenchymal stem cells (MSCs). Bone marrow of healthy monkey ilium was extracted, mononuclear cells were isolated, inoculated in L-DMEM culture medium containing 15% FBS (bFGF, 3 ng / ml was added therein), and cultured in plastic culture flasks. The 5th to 8th generation bone marrow MSCs were taken for identification. The methods included flow cytometry to detect their surface antigen markers, and in vitro induction to observe their osteogenic and adipogenic multidirectional differentiation potential. 48 hours before transplantation, Brdu was labeled at a concentration of 10umol / L and counted for later use.
[0034] (average)
[0035] It can be seen from the results that 1.5 hours of induction can not only ensure the effect of induction but also ensure the activity of cells, which is the best induction time. At present, the transplantation of induced cells does not pay attention to the activ...
Embodiment 4
[0038]Example 4. Experimental transplantation of bone marrow MSCs-derived neural precursor cells and GDNF controlled-release biomaterials to treat monkey neurological dysfunction caused by artificial spinal cord injury. According to the methods described in Example 1 and Example 2, MSCs were isolated and cultured and GDNF controlled-release biomaterials were prepared. Animal models were prepared, and 8 healthy male rhesus monkeys weighing 4 kg were randomly divided into groups. Combined anesthesia with intramuscular ketamine hydrochloride injection and diazepam injection was used to make the thoracic spinal cord injury model according to the modified Allen's method.
[0039] The experimental design is as follows:
[0040] Transplantation group: on the 10th day after injury, digest and count the induced monkey MSCs, divide 3.0×10 6 200uL of PBS suspension made from cells / kg and controlled-release biomaterial containing 2ugGDNF, injected into the experimental group (n=4) at fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com