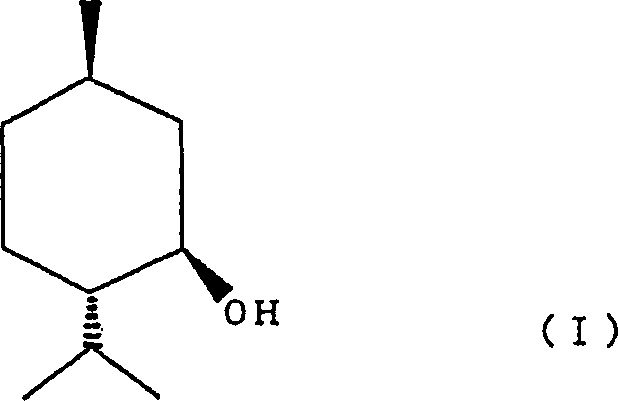
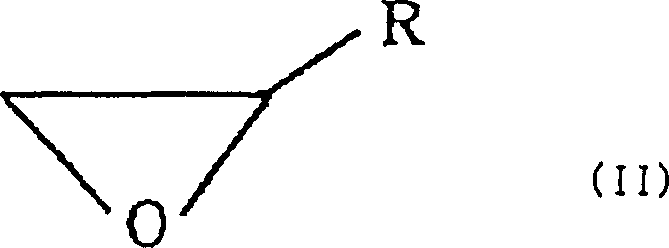
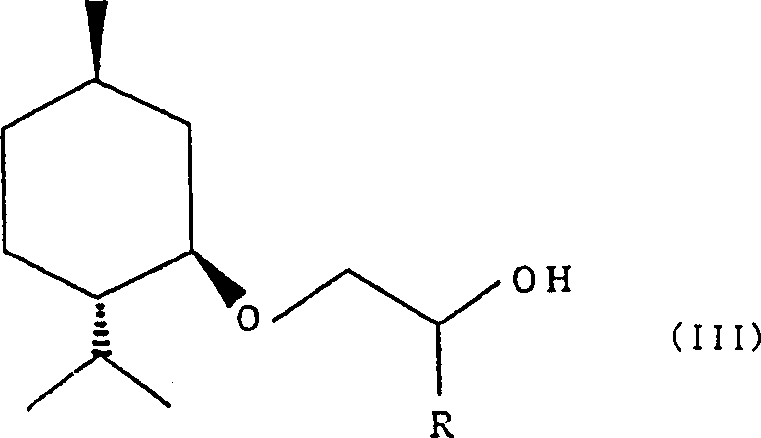
Process for producing 2-(l-menthoxy)ethanol compound
A technology of menthyl oxygen and compounds, which is applied in the field of preparation of 2-ethanol compounds, can solve the problems of not being cheap, cumbersome procedures, and high costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of 2-(L-menthyloxy)ethanol using anhydrous aluminum chloride
[0036] (1) In a 1000ml four-neck flask, add 100.0g (641mmol) of L-menthol and 42.7g (320mmol) of anhydrous aluminum chloride, replace with nitrogen, add 500ml of toluene while ice-cooling and start stirring. After confirming that the temperature of the reaction liquid had dropped to about 5°C, 57 g of ethylene oxide was added while maintaining the liquid temperature at 5 to 10°C. After the feed was complete, further stirring was carried out at room temperature for 1 hour to complete the reaction.
[0037] (2) Next, the reaction solution was cooled again and washed with 150 ml of 10% hydrochloric acid while keeping the temperature below 20°C. Next, wash with 50 ml of saturated sodium bicarbonate and 100 ml of saturated brine, remove water with sodium sulfate, and recover the solvent under reduced pressure to obtain 123.1 g of crude 2-(L-menthoxy)ethanol. Carry out Vigoreux distillatio...
Embodiment 2
[0045] Example 2: Preparation of 2-(L-menthoxy)ethanol using various Lewis acid catalysts
[0046] With respect to the L-menthol of 1mol, respectively use the Lewis acid catalyst shown in table 2 below 0.2mol, adopt the reaction temperature and the reaction time as shown in table 2, other conditions are the same as embodiment 1, make L-menthol and Ethylene oxide reacts to produce 2-(L-menthoxy)ethanol. The results are shown in Table 2 below, and the conversion, selectivity and yield have the same meanings as in Table 1.
[0047] Table 2
[0048] experiment
Catalyst (Reagent)
temperature reflex
(℃)
Reaction time
(hr)
Conversion rates
(%)
selectivity
(%)
Yield
(%)
type
Usage amount 1)
1
Anhydrous AlCl 3
0.2mol
40
2.0
38.8
86.2
33.5
2
ZnCl 2
0.2mol
40
3.0
27.4
91.2
25.0
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com