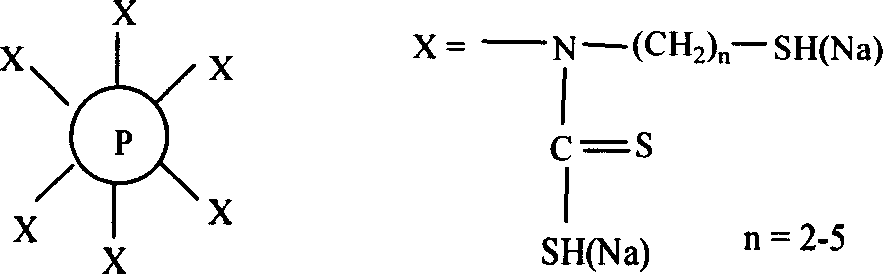Novel high effective noble metal adsorbent and its preparing method
An adsorbent and precious metal technology, applied in the field of novel high-efficiency precious metal adsorbent and its preparation, can solve the problems of complex preparation, difficult market application of adsorbent resin, and many synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
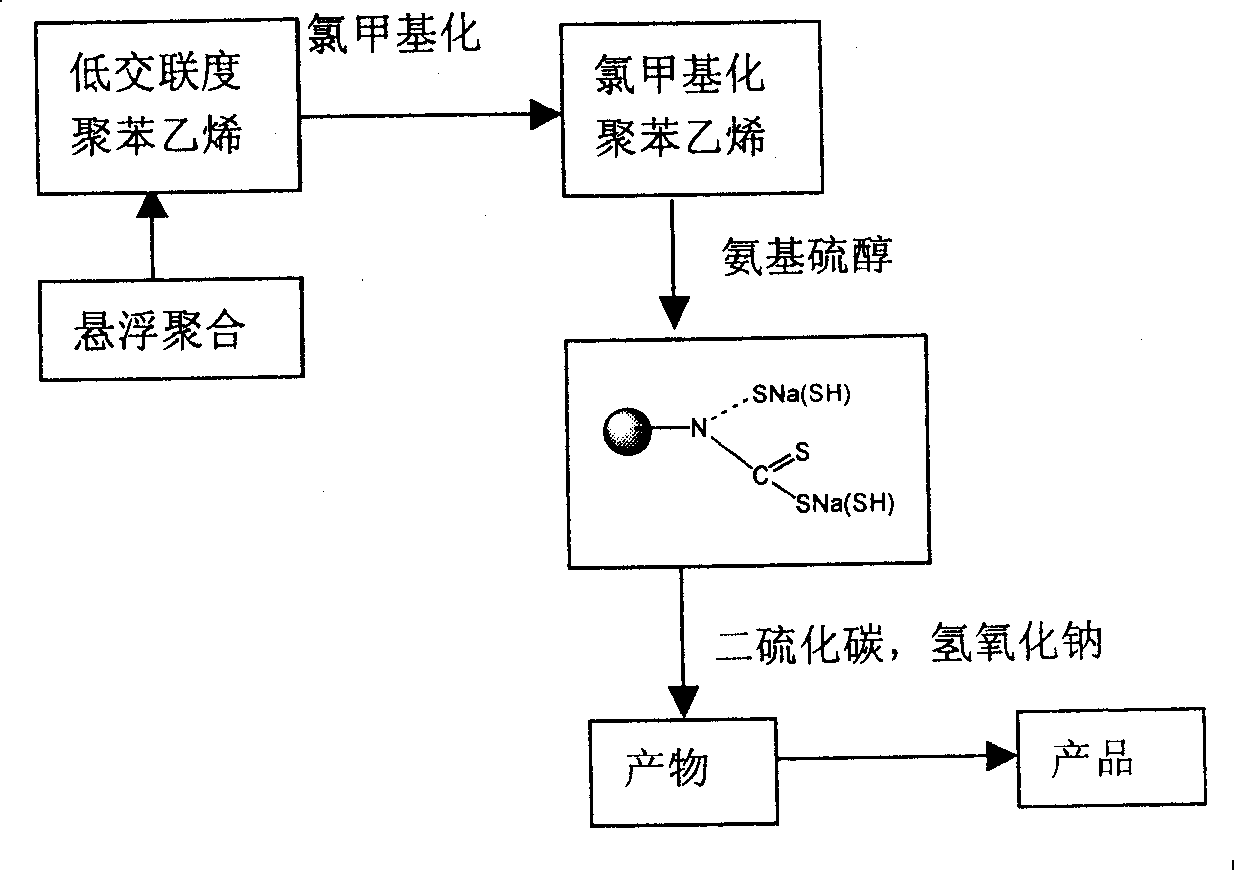
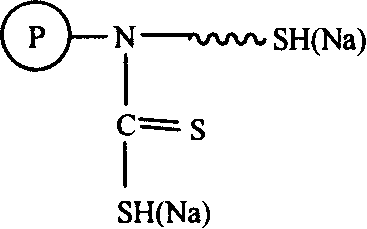
[0021] (1) Weigh 40.0 g of low-crosslinking polystyrene resin prepared by suspension polymerization, swell in 0.15 L of ethanol, and react with chloromethyl ether to realize chloromethylation. The introduction of the chloromethyl group was characterized by NMR. Characterize the chlorine content with elemental analysis; (2) react chloromethyl with aminoethanethiol (50% aqueous solution, 70mL), adjust the pH value to 7, and the reaction temperature is 40~60°C; (3) take the above reaction product 20.0 g, join CS 2 (100.0g) and NaOH aqueous solution (10%, 40mL) continued to react for 4 hours, the product was taken out, filtered, acid washed, washed with water, and dried to obtain the adsorbent. The product is characterized by nuclear magnetic resonance, infrared spectroscopy, and elemental analysis. Its basic structural formula is:
[0022]
[0023] Among them, the surface of styrene beads can carry multiple adsorption groups, and the left figure is only a schematic diagram. ...
Embodiment 2
[0026] (1) Get 40.0 g of low-crosslinking polystyrene resin obtained by suspension polymerization, swell in N, N'-dimethylformamide (0.10 L) and react with chloromethyl ether to realize chloromethylation; (2 ) chloromethyl reacts with aminoethanethiol (50% ethanol solution, 75mL), adjusts the pH value to 7, and the reaction temperature is 40-60°C; (3) Take 20.0g of the above reaction product, add CS 2 (80.0g) and NaOH aqueous solution (5%, 60mL) continue to react after 4 hours, take out product, filter, pickle, wash with water, dry to obtain product (same as embodiment 1).
[0027] The adsorbent adsorbs gold solutions with different pH values (4-10), and then desorbs, and measures the gold purity after desorption. Experiments show that under different pH values, the amount of gold adsorbed in the electroplating solution wastewater is basically the same, the gold purity is 99%, and the resin can be reused.
Embodiment 3
[0029] (1) Take 40.0g of polystyrene resin, swell in dioxane (0.13L) and react with chloromethyl ether to realize chloromethylation; (2) chloromethyl and aminopropanethiol (50% aqueous solution, 75mL) reaction, adjust the pH value to 7, the reaction temperature is 40 ~ 60 ℃; (3) take 20.0g of the above reaction product, add CS 2 (80.0g) and NaOH aqueous solution (5%, 60mL) continue to react after 4 hours, take out product, filter, pickle, wash with water, after drying, obtain product (same as embodiment 1). The resin adsorbs gold solutions with different pH values (4-10), and then desorbs, and measures the gold purity after desorption. Experiments show that under different pH values, the amount of gold adsorbed in the electroplating solution wastewater is basically the same, the gold purity is 99%, and the resin can be reused.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com