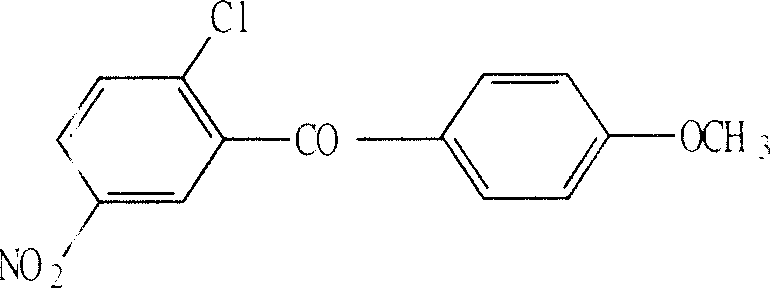
Process for preparing 2-chloro-5-nitro phenyl -4'-methoxy benzophenone
A technology of methoxybenzophenone and nitrophenyl, which is applied in the field of preparation of 2-chloro-5-nitrophenyl-4'-methoxybenzophenone, can solve complicated operations and difficult product separation , low yield and other problems, to achieve the effect of high content, good product purity and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
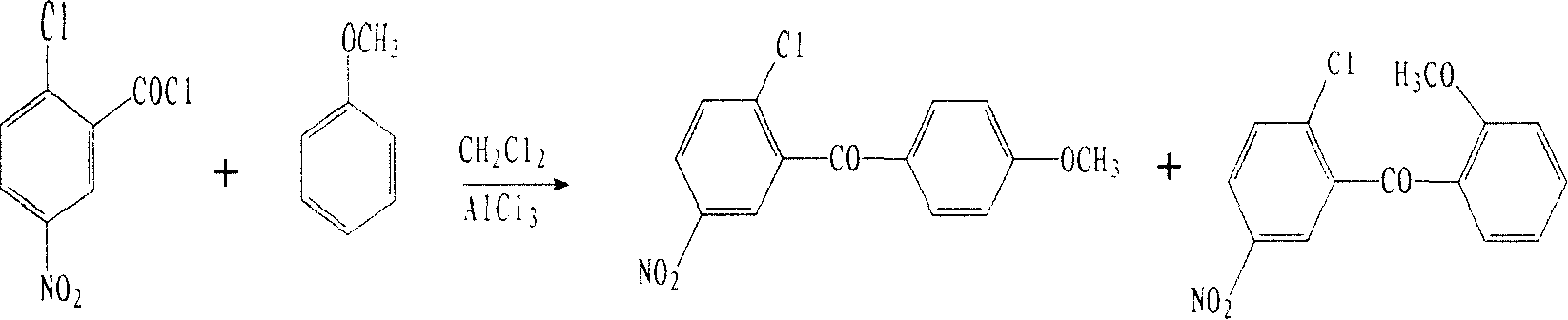
[0022] Add 500ml of dichloromethane to a 1000ml four-necked flask equipped with a stirrer and a thermometer, and add 87.4g (0.3969mol) of 2-chloro-5-nitrobenzoyl chloride 72.0g (0.6658mol) of anisole under stirring, and the reaction solution Cool to -8°C ~ -12°C, add 72.0g (0.5560mol) of anhydrous aluminum trichloride, maintain the temperature, react for 8 hours; drop 240ml of 10% hydrochloric acid in 2 hours, let stand overnight; separate the water phase , dichloromethane solvent was distilled off, ethyl acetate / ethanol (V:V=1:1) mixed solvent 350ml was added to the residue, heated to reflux for 2 hours, cooled, white crystals were precipitated, filtered and dried to obtain the target product 2 -Chloro-5-nitrophenyl-4'-methoxybenzophenone 68.5g, yield 59.1%, analysis result: purity ≥ 98.2% (HPLC), melting point 111.1 ° C ~ 112.7 ° C (literature value: 111 ~ 113 ℃), NMR identification structure:
[0023] 1 H NMR ([D 6 ]DMSO 400MHz)
[0024] δ: 3.87 (s, 3H) δ: 6.90 (d, 2H) ...
Embodiment 2
[0027] In a 1000ml four-necked flask equipped with a stirrer and a thermometer, add 500ml of methylene chloride, add 87.4g (0.3969mol) of 2-chloro-5-nitrobenzoyl chloride, 80.0g (0.7398mol) of anisole under stirring, Cool the reaction solution, add 75.0g (0.5792mol) of anhydrous aluminum trichloride at -10°C to -15°C, and maintain the temperature for 15 hours; add 220ml of 10% hydrochloric acid dropwise within 2 hours, and let it stand overnight; phase, evaporate the dichloromethane solvent, add 500ml ethyl acetate / ethanol (V:V=1:1) mixed solvent to the residue, heat and reflux for 2 hours, cool, and precipitate white crystals, filter, and dry to obtain 2- Chloro-5-nitrophenyl-4'-methoxybenzophenone 71.1g, yield 61.4%, analysis result: purity ≥ 98.5% (HPLC), melting point 111.3°C-112.7°C.
Embodiment 3
[0029] Add 55 l of methylene chloride in a 100 l reactor equipped with a stirrer and a thermometer, add 8.74kg (39.68mol) of 2-chloro-5-nitrobenzoyl chloride, 7.5kg (69.35mol) of anisole under stirring , the reaction solution was cooled, and 7.2kg (55.60mol) of anhydrous aluminum trichloride was added at -8°C to -12°C, and the temperature was maintained for 10 hours; 24 liters of 10% hydrochloric acid was added dropwise within 2 hours, and allowed to stand overnight; Remove the water phase, evaporate the dichloromethane solvent, add 40 l of ethyl acetate / ethanol (V:V=1:1) to the residue, heat to reflux for 2 hours, cool, and precipitate white crystals, filter, and dry 7.01 kg of 2-chloro-5-nitrophenyl-4'-methoxybenzophenone was obtained with a yield of 60.5%. Analysis results: purity ≥ 98.5% (HPLC), melting point 111.2°C-112.7°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com