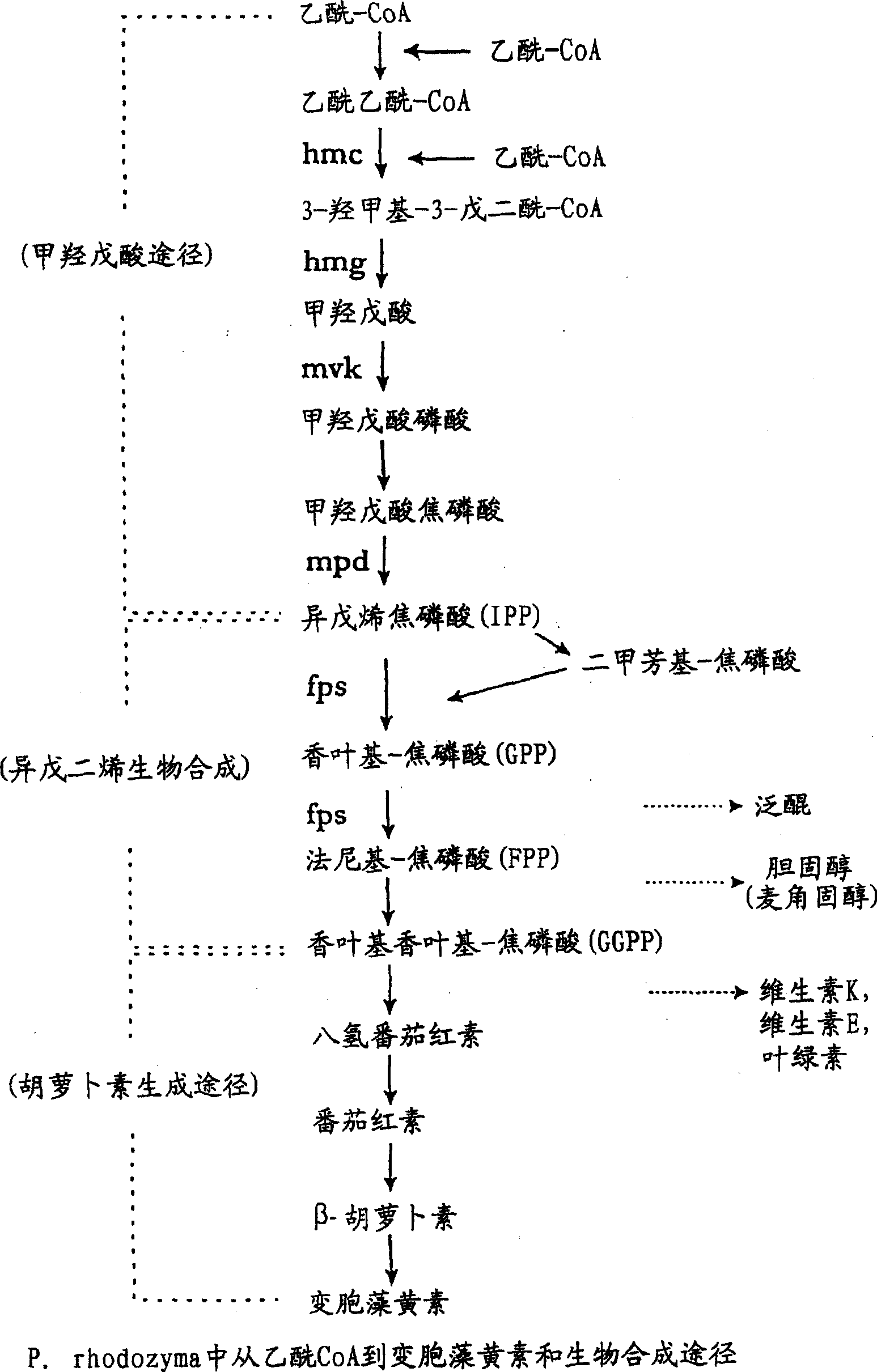
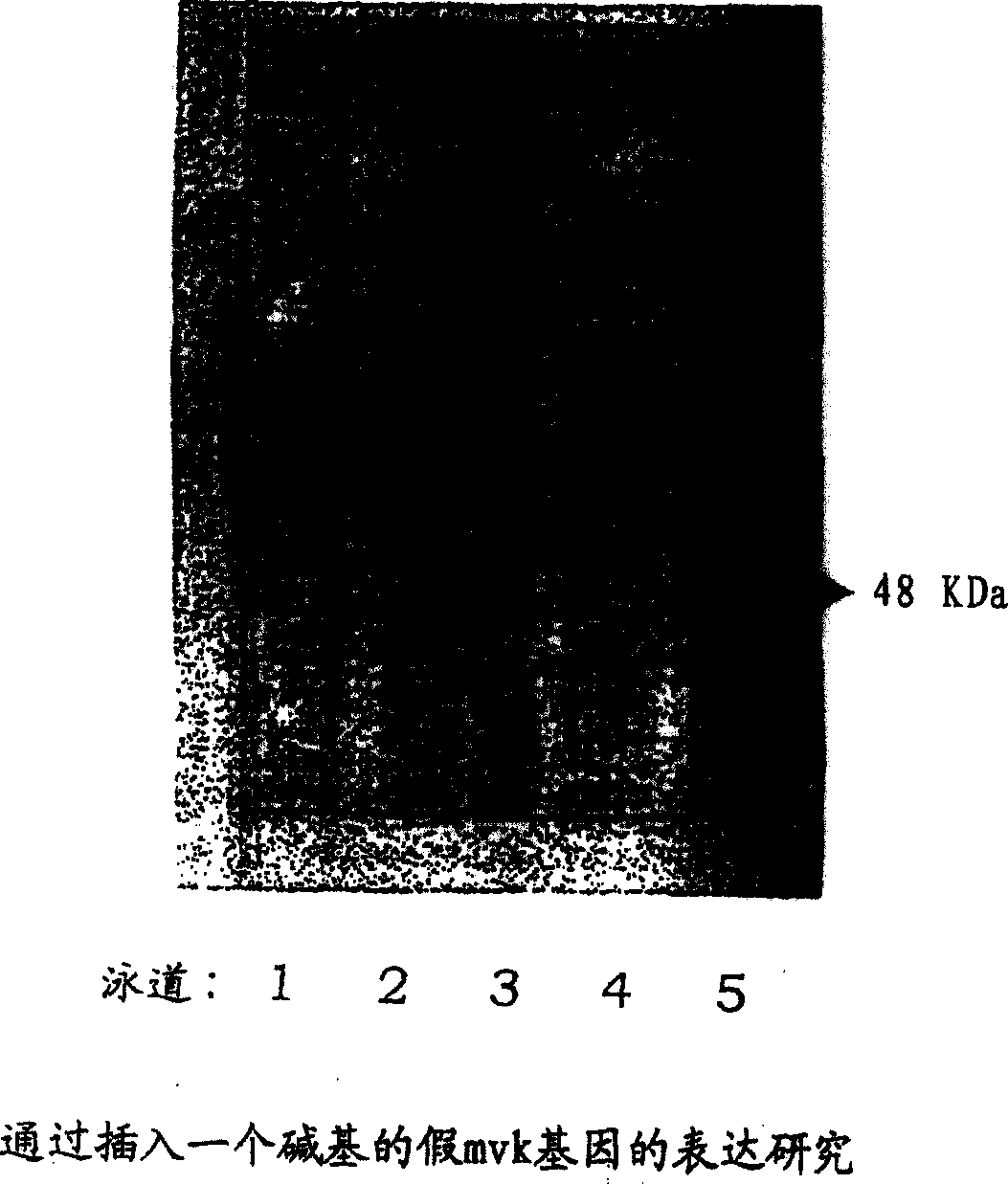
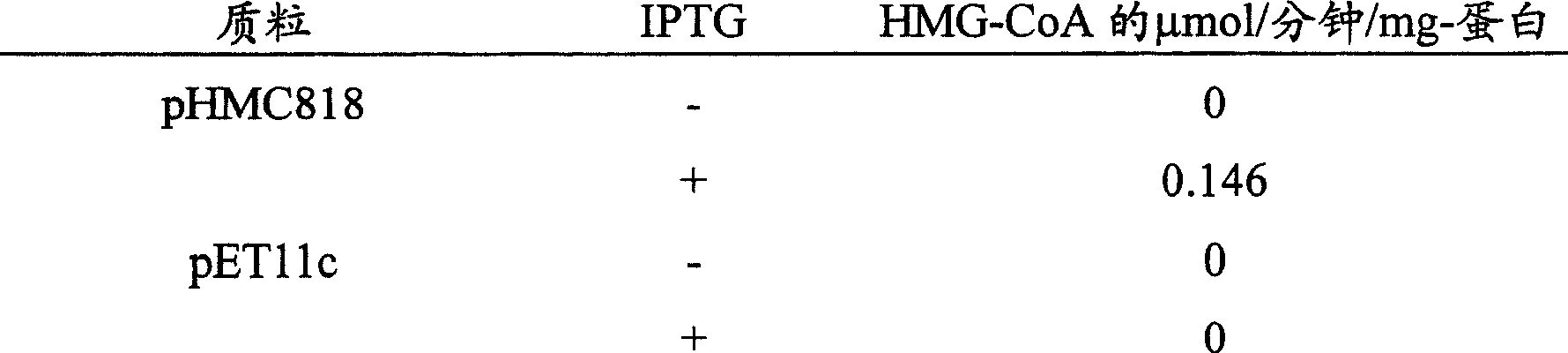
Improved production of isoprenoids
A kind of technology from isopentenyl pyrophosphate and methylglutaryl, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Isolation of mRNA of P.rhodozyma and construction of a cDNA library
[0084] To construct the cDNA library of P. rhodozyma, total RNA was isolated by phenol extraction immediately after cell disruption and the mRNA of P. rhodozyma ATCC96594 was purified using an mRNA preparation kit (Clontech).
[0085] First, cells of the ATCC 96594 strain in 2-day cultures in 10 ml of YPD medium were harvested by centrifugation (1500×g, 10 minutes) and washed once with extraction buffer (10 mM sodium citrate / HCl (pH 6.2), containing 0.7 M HCl). After being suspended in the extraction buffer of 2.5ml, use a French press homogenizer (Ohtake Works Corp., Japan) at 1500kgf / cm 2 Cells were disrupted and immediately mixed with twice the volume of isogen (Nippon gene) according to the manufacturer's recommendation. In this step, 400 μg of total RNA was recovered.
[0086] Total RNA was then purified using the mRNA Isolation Kit (Clontech) as described by the manufacturer. Final...
Embodiment 2
[0088] Example 2 Cloning of part of the hmc (3-hydroxy-3-methylglutaryl-CoA synthase) gene of P. rhodozyma
[0089] To clone part of the hmc gene of P. rhodozyma, a degenerate PCR method was used. Based on the consensus sequences from known HMG-CoA synthase genes with other species, the nucleotide sequences of two mixed primers were designed and synthesized as shown in Table 1.
[0090] Table 1
[0091] Primer sequences for cloning hmc gene
[0092] Hmgsl GGNAARTAYACNATHGGNNYTNGGNCA (sense primer) (SEQ ID NO: 11)
[0093] Hmgs3 TANARNSNNSWNGTRTACATR TINCC (antisense primer) (SEQ ID NO: 12) CN=A, C, G or T, R=A or G, Y=C or T, H=A, T or C, S=C or G , W=A or T)
[0094] After 25 cycles of PCR reaction at 95° C. for 30 seconds, 50° C. for 30 seconds, and 72° C. for 15 seconds using ExTaq (Takara Shuzo) as a DNA polymerase and the cDNA library obtained in Example 1 as a template, the reaction mixture was Perform agarose gel electrophoresis. PCR bands of the desired length we...
Embodiment 3
[0095] Example 3 Isolation of Genomic DNA of P. rhodozyma
[0096] To isolate the genomic DNA of P. rhodozyma, the QIAGEN genomic kit was used according to the manufacturer's protocol.
[0097] First, cells of P. rhodozyma ATCC 96594 strain in 100 ml YPD medium overnight culture were harvested by centrifugation (1500×g, 10 minutes) and washed once with TE buffer (10 mM Tris / HCl (pH 8.0), containing ImM EDTA). After suspending in 8ml Y1 buffer of QIAGEN genome kit, lysozyme was added at a concentration of 2mg / ml to enzymatically break down the cells and the reaction mixture was incubated at 30°C for 90 minutes, followed by the next step of extraction. Finally, 20 μg of genomic DNA was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com