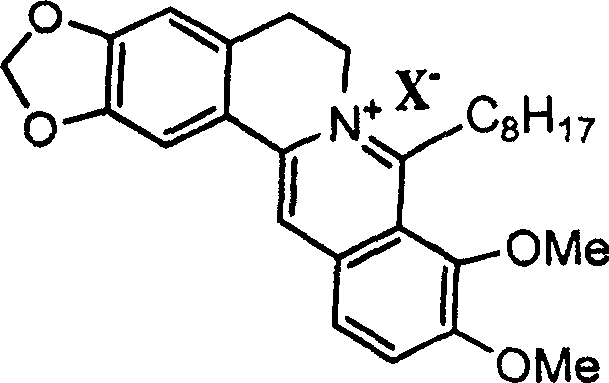
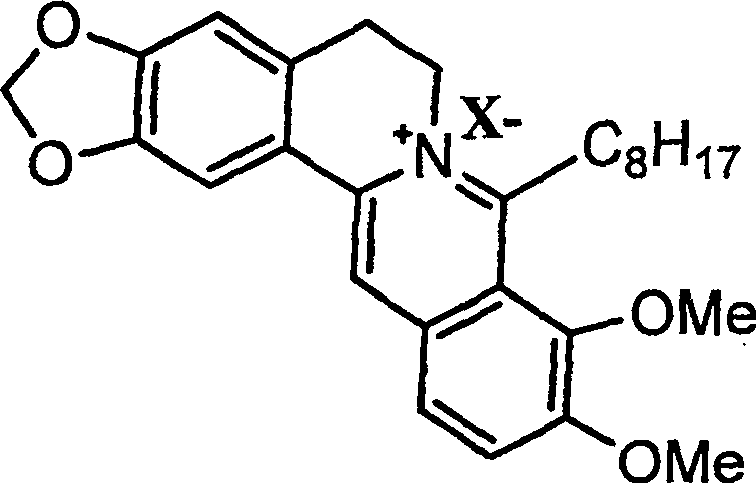
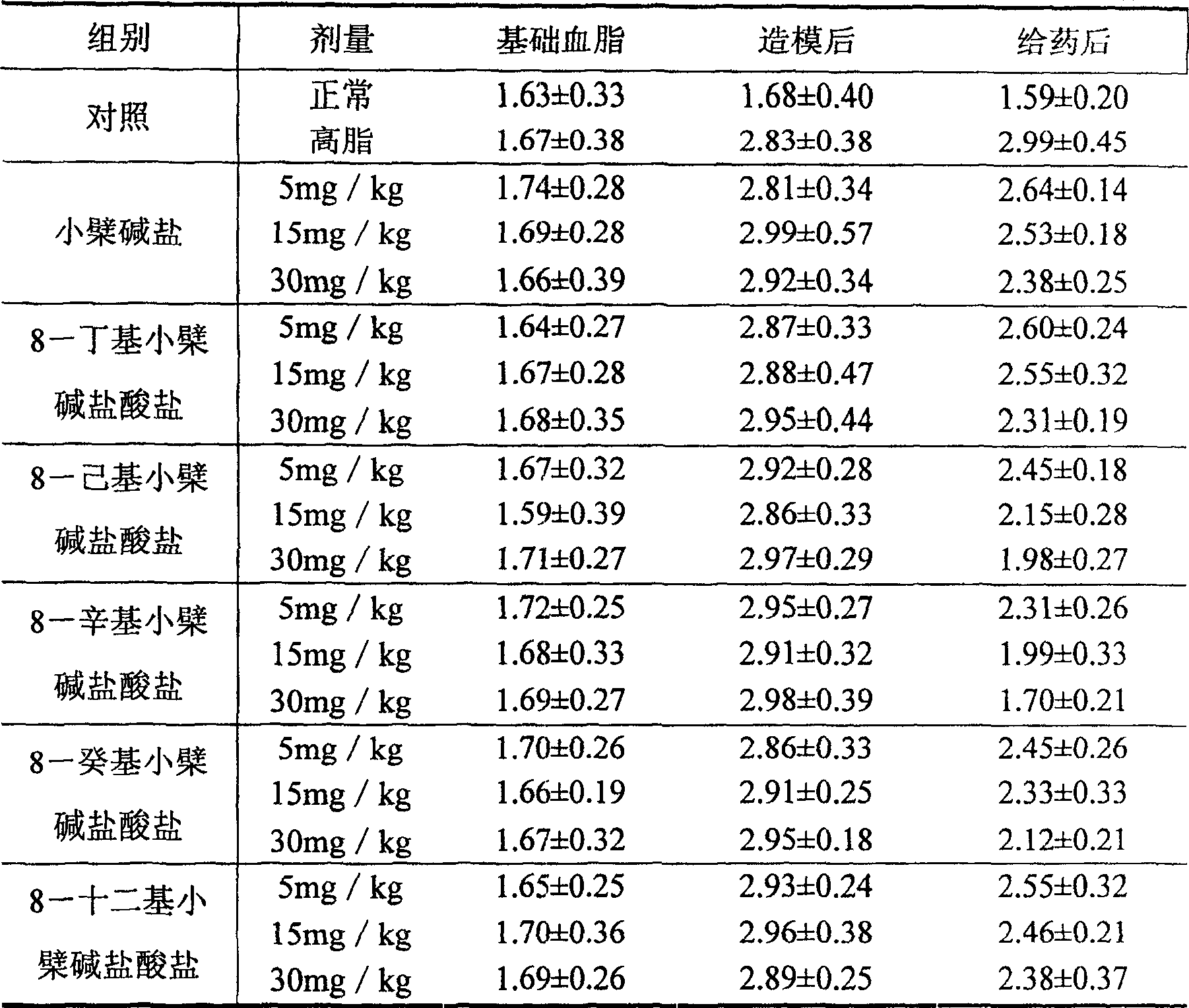
8-octyl berberine hydrochloride and its synthesis process and application
A technology of berberine salt and berberine hydrochloride, which is applied in the directions of drug combination, metabolic diseases, organic chemistry, etc., can solve the 8-position of 8-alkyl berberine salt that has not been reported and has not been seen yet. Connected to problems such as long-chain alkyl groups, to achieve the effect of good development and utilization value, low price and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of 8-octylberberine hydrochloride
[0036] ①. Dry all reaction glass instruments, weigh 0.1mol of dried magnesium chips and place in a 250mL three-necked flask, use 40mL of anhydrous tetrahydrofuran as the reaction solvent, add 0.1mol of n-octane chloride under nitrogen protection, and prepare the corresponding Grignard reagent.
[0037] ②. Weigh 0.1mol of dry berberine hydrochloride and place it in a 500mL three-neck flask, add 100mL of anhydrous tetrahydrofuran to make a suspension of berberine hydrochloride, and then place it in an ice bath to -10°C under nitrogen protection.
[0038] ③. Slowly add the prepared Grignard reagent to the berberine suspension under nitrogen protection and ice bath, while stirring. After the addition, remove the ice bath, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0039] ④, centrifuge the reaction solution, take the supernatant, then add tetrahydrofuran to extract, rep...
Embodiment 2
[0043] ①. Dry all reaction glass instruments, weigh 0.12mol of dried magnesium chips and place in a 250mL three-necked flask, use 100mL of anhydrous ether as the reaction solvent, add 0.06mol of n-bromodecane under nitrogen protection, and prepare the corresponding Grignard reagent.
[0044] ②. Weigh 0.03mol of dry berberine hydrochloride and place it in a 500mL three-neck flask, add 100mL of anhydrous ether to form a suspension of berberine hydrochloride, and then ice-bath to 0°C under nitrogen protection.
[0045] ③. Slowly add the prepared Grignard reagent to the berberine suspension under nitrogen protection and ice bath, while stirring. After the addition, remove the ice bath, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0046] ④, centrifuge the reaction solution, take the supernatant, then add diethyl ether to extract, repeat several times, monitor the extraction situation of the reaction product with the thin layer method, until...
Embodiment 3
[0050] ①. Dry all reaction glass instruments, weigh 0.12 mol of dried magnesium chips, put 15 L of oxygen six in a 250 mL three-necked flask as the reaction solvent, add 0.1 mol of n-dodecane iodide under nitrogen protection, and prepare the corresponding Grignard reagent.
[0051] ②. Weigh 0.05mol of dry berberine hydrochloride and place it in a 500mL three-necked flask, add 100mL of dioxane to form a suspension of berberine hydrochloride, and then ice-bath to 10°C under nitrogen protection.
[0052] ③. Slowly add the prepared Grignard reagent to the berberine suspension under nitrogen protection and ice bath, while stirring. After the addition, remove the ice bath, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0053] ④, centrifuge the reaction solution, take the supernatant, then add dioxane to extract, repeat several times, monitor the extraction situation of the reaction product with the thin layer method, until the raw material poi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com