Liquid crystal mixture
A mixture and liquid crystal technology, applied in liquid crystal materials, organic chemistry, instruments, etc., can solve the problem of narrowing the range of nematic liquid crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0226] Preparation of components
[0227] A solution of LQT 120 (Hitachi Kasei) was spin-coated at 2500 rpm on a glass substrate with indium tin oxide (ITO). The substrate was heated at 200°C for 1 hour to form a thin film. After wiping the coated film with a nylon cloth in one direction, the substrates were assembled into a cell, and a gasket with a thickness of 2.0 μm was inserted between the substrates in such a way that the directions of wiping were antiparallel to each other. Fill the liquid crystal mixture into the pool in the isotropic phase stage, gradually cool down through the nematic phase, (pass through the smectic A phase when the liquid crystal mixture has a smectic A phase) and the smectic C phase, and then cool at 25°C The performance of the liquid crystal mixture was tested by applying a rectangular wave pulse (60 Hz) to the cell with varying applied voltage (0-10 V).
[0228] Resistivity and ion-induced spontaneous polarization were measured with an MTR-1 d...
Embodiment 7
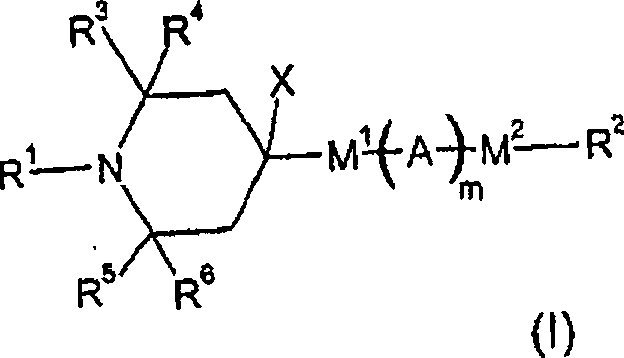
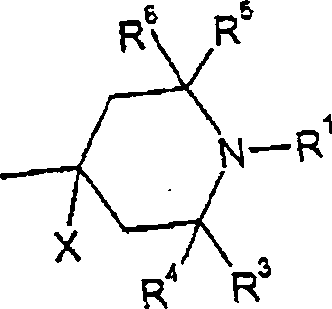
[0233] 2,2,6,6-Tetramethyl-piperidine-4-(4-octyloxy)benzoate is prepared by using 4-octyloxybenzoyl chloride to make 4-hydroxy-2,2,6,6 -Tetramethylpiperidine is obtained by esterification, and the preparation method is similar to that described in US-A-4038280; the melting point is 79-81°C.
Embodiment 8
[0235] 2,2,6,6-tetramethyl-piperidine-4-(4'-octylbiphenyl-4-yl) carboxylate was obtained from 4'-octylbiphenyl- 4-base formyl chloride in the system; melting point 104-106 ° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com