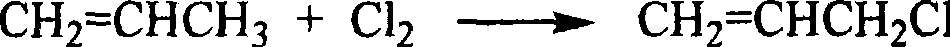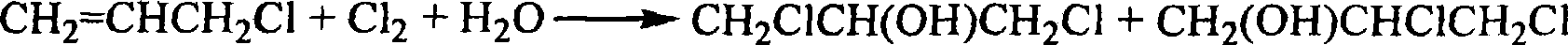Preparation method of dichloro propanol from glycerin
A technology of dichloropropanol and glycerin, which is applied in the chemical industry, can solve problems such as low reaction efficiency, low equipment utilization rate, and easy coke formation, and achieve the effects of reduced energy consumption, increased equipment utilization rate, and increased reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
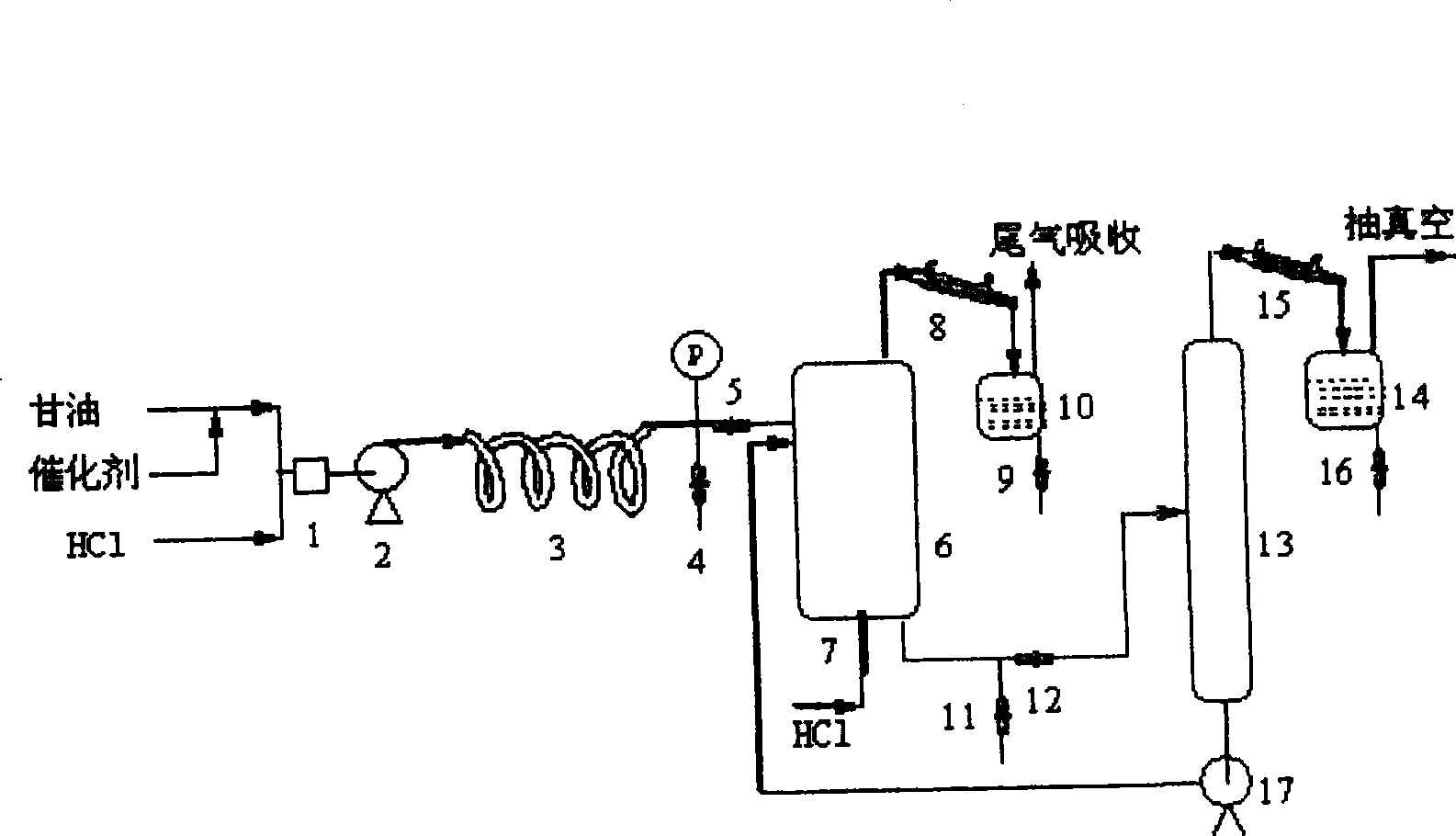
[0040] Example 1. Glycerol (95% industrial product) is mixed with HCl (by PCl 3 with H 2 O is produced by 1:3.5 reaction) at 0.6mol / h. Pumped in via pre-mixing figure 1In the reaction system shown, the temperature of the oil bath in the tubular reactor is 105°C, which is adjusted by the outlet flow regulating valve 5, so that the residence time of the material in the tubular reactor is 6h. Gas chromatographic analysis through the sampling valve 4 shows that the conversion rate of glycerin is 93.6%, the generation rate of monochloropropanediol is 84.4%, and the generation rate of dichloropropanol is 8.9%. NaOH standardizes the acid concentration of the reaction solution to 0.49mol / L.
[0041] The reaction liquid flowing out of the tubular reactor directly enters the HCl bubble column reactor, the reaction temperature is controlled at 108°C, the feed rate of HCl is 1.2mol / h, and the flow rate of the material in the bubble reactor is controlled through the outlet valve 12. Th...
example 2
[0043] Example 2. Changing the residence time of materials in the bubble reactor. Put industrial glycerin (95%) at 0.6 mol / h (which contains 2% acetic acid as a catalyst) and HCl at 0.6 mol / h through pre-mixed pumping figure 1 In the reaction system shown, the temperature of the oil bath in the tubular reactor is 100°C, and the outlet pressure and flow rate are adjusted, and the residence time of the material in the tubular reactor is 6h.
[0044] Control the temperature of the bubbling reactor to 110°C, adjust the outlet flow rate, so that the residence time of the material in the bubbling reactor is 7h, the amount of HCl introduced is 0.8mol / h, and the generated azeotrope is condensed with the tail gas. Liquid separation and collection.
[0045] Within 5 hours of steady-state operation, 295 g (3 mol) of glycerol was fed, 9 mol of HCl was fed, and glycerol was completely converted. 198 g of condensate (including 39.1 g of dichloropropanol, 62 g of HCl, 91.8 g of water, and ...
example 3
[0046] Example 3. Changing the residence time of the material in the tubular reactor. Industrial glycerin (95%) is fed with 1.0mol / h (containing 2% acetic acid), HCl 1.0mol / h, and the temperature of the tubular reactor is controlled at 105°C. The residence time of the material in the tubular reactor is about 3.5h. The conversion rate of glycerol at the outlet of the formula reactor was 81.6%, the production rate of monochloropropanediol was 79.2%, and the production rate of dichloropropanol was 2.0%. The acid value of NaOH is 1.4mol / L.
[0047] The reaction liquid in the tubular reactor enters the HCl bubbling reactor to continue the reaction, the HCl feed rate is 2mol / h, the temperature of the bubbling reactor is controlled at 110°C, and the residence time is 10h.
[0048] Within 5 hours of steady-state operation, 5 mol of glycerol and 15 mol of HCl were fed, and glycerol was completely converted. Condensate 257.2g of condensate from the tail gas of the bubble reactor, whic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com