M-benzenedicarbonyl thioureas derivative, preparation and application thereof as fluorinion identification receptor
A technology of isophthaloyl thiourea and isophthaloyl chloride, applied in the field of fluoride ion recognition receptors, can solve the problems of environmental pollution, solvent volatilization waste, flammability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
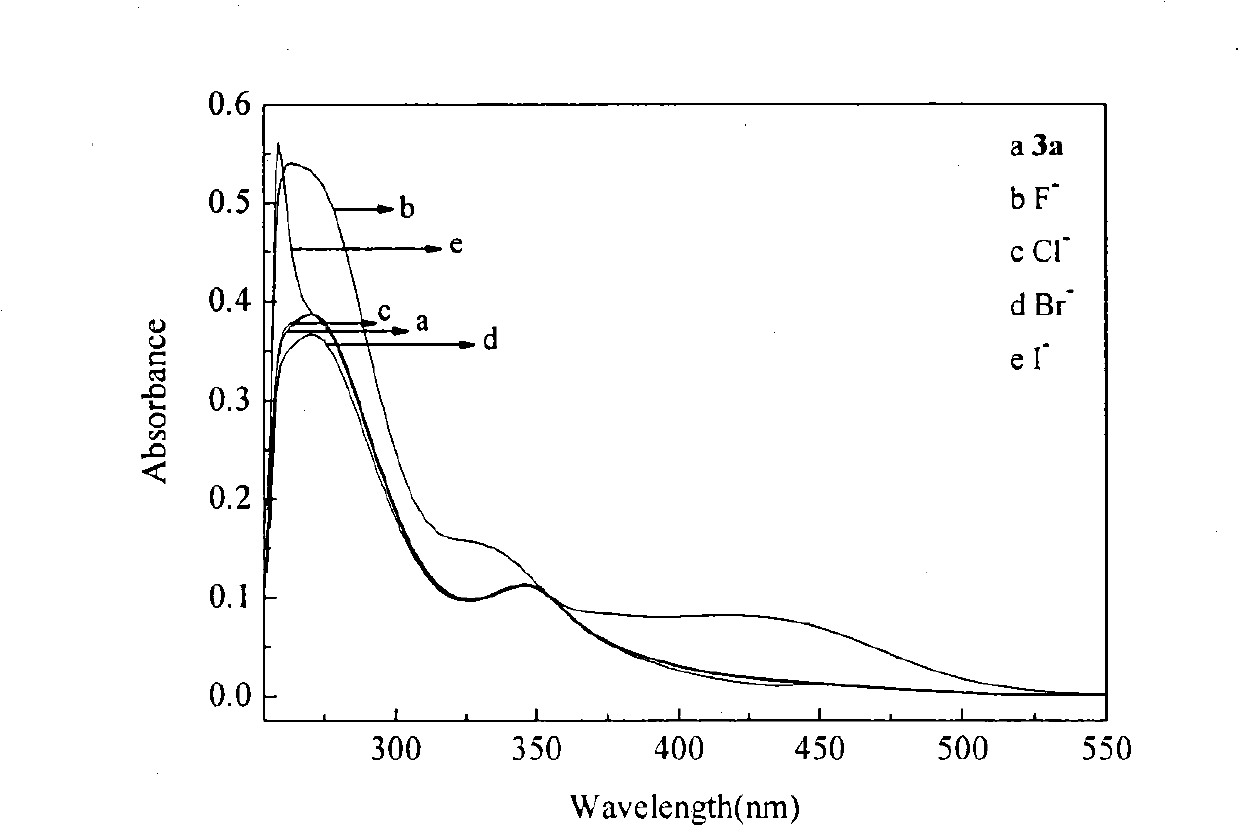
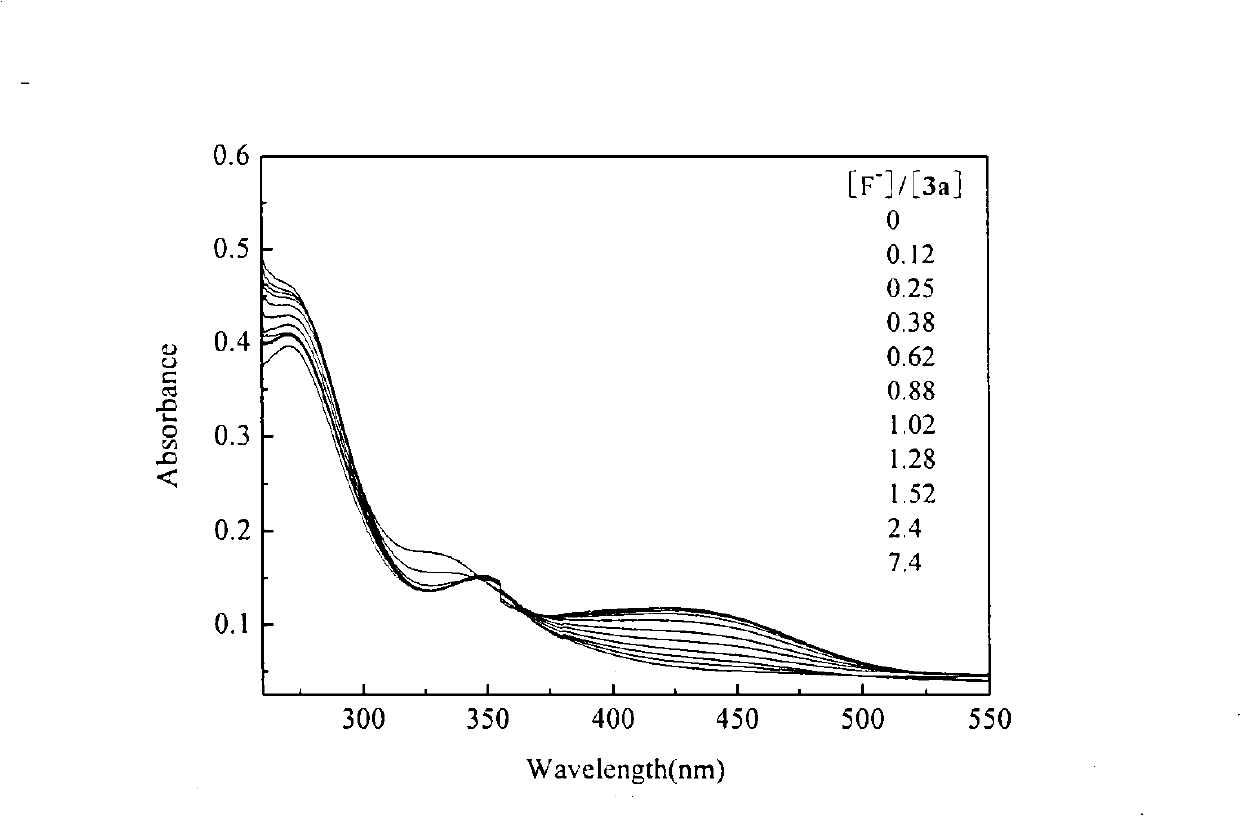
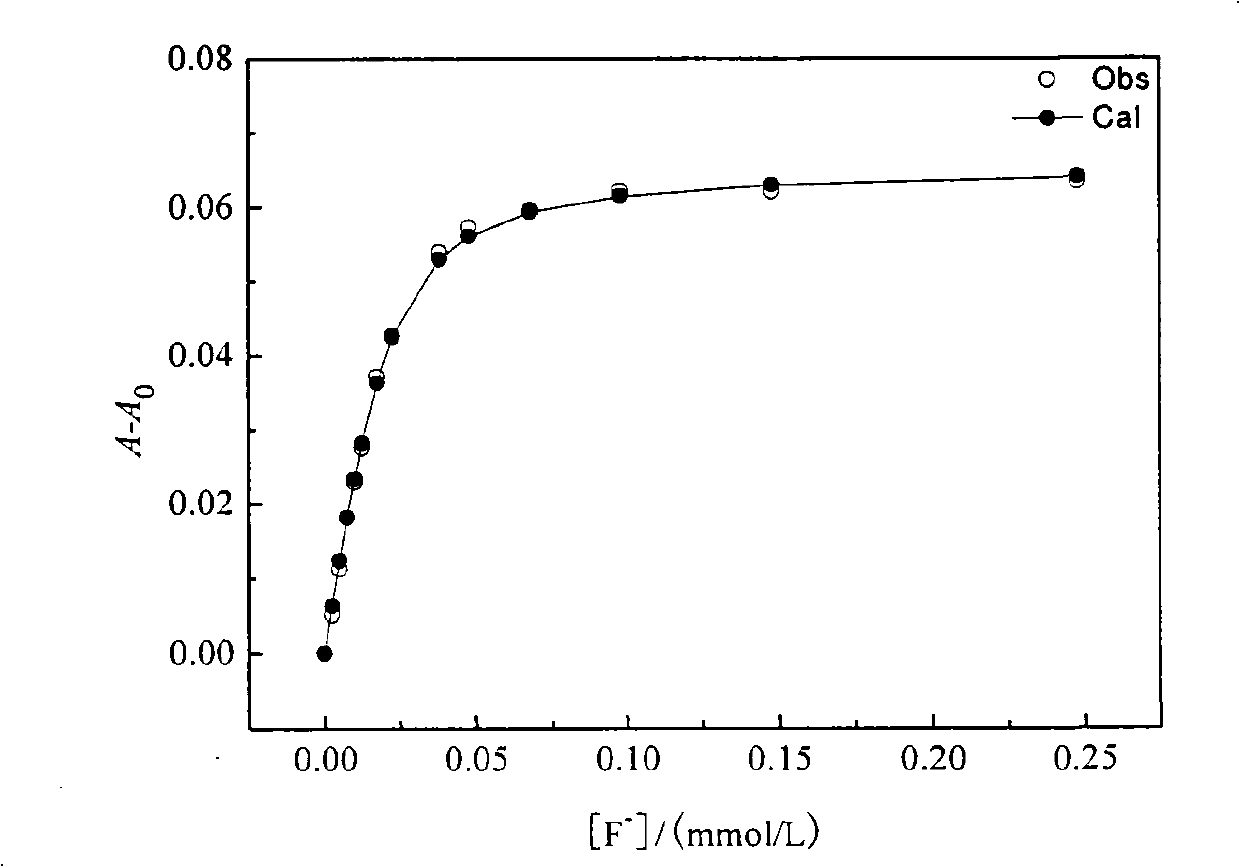
[0063] Embodiment 1, the preparation method of isophthaloyl thiourea derivative 3a of the present invention: be that isophthaloyl dichloride and ammonium thiocyanate are joined in the mortar with the molar ratio of 1: 2.5, then add thiocyanic acid Polyethylene glycol-400 with an ammonium content of 2% was intermittently ground and reacted for 5 hours at room temperature to obtain isophthaloyl isothiocyanate; base aniline, reacted intermittently at room temperature for 6 hours to obtain a yellow precipitate; filtered, washed, and washed with DMF-C 2 h 5 OH-H 2 O (where DMF:C 2 h 5 OH:H 2 O (the molar ratio of O is 1:0.8:0.4) was recrystallized from a mixed solvent to obtain the isophthaloylthiourea derivative 3a with a yield of 72.4%.
Embodiment 2
[0064] Embodiment 2, the preparation method of isophthaloyl thiourea derivative 3b of the present invention: be that isophthaloyl dichloride and ammonium thiocyanate are joined in the mortar with the molar ratio of 1: 2.8, then add thiocyanic acid Polyethylene glycol-400 with an ammonium content of 3% was intermittently ground and reacted for 6 hours at room temperature to obtain isophthaloyl isothiocyanate; then add aniline with 2.2 times the amount of isophthaloyl chloride, Intermittent grinding reaction at room temperature for 24 hours, a pale yellow precipitate was obtained; filtered, washed, and washed with DMF-C 2 h 5 OH-H 2 O (where DMF:C 2 h 5 OH:H 2 O (the molar ratio of O is 1:1.0:0.5) was recrystallized from a mixed solvent to obtain the isophthaloylthiourea derivative 3b with a yield of 87.5%.
Embodiment 3
[0065] Embodiment 3, the preparation method of isophthaloyl thiourea derivative 3c of the present invention: be that isophthaloyl dichloride and ammonium thiocyanate are joined in the mortar with the molar ratio of 1: 3, then add thiocyanic acid Polyethylene glycol-400 with an ammonium content of 4% was ground intermittently for 7 hours at room temperature to obtain isophthaloyl isothiocyanate; then add 2.5 times the amount of isophthaloyl chloride Oxyaniline, reacted intermittently at room temperature for 48 hours to obtain a pale yellow precipitate; filtered, washed, and washed with DMF-C 2 h 5 OH-H 2 O (where DMF:C 2 h 5 OH:H 2 O (the molar ratio of O is 1:1.2:0.6) was recrystallized from a mixed solvent to obtain the isophthaloylthiourea derivative 3c with a yield of 84.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com