Electroluminescent metallo-supramolecules with terpyridine-based groups
A supramolecular and metal technology, applied in luminescent materials, 2/12 group organic compounds without C-metal bonds, circuits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of model compound 5a - heating zinc acetate dihydrate (1 mmol) and 4'-phenyl-2,2':6' in 10 ml of N-methylpyrrolidone (NMP) under nitrogen atmosphere at 100 °C , 2"-terpyridine (1mmol) for 3 hours. After filtration, an excess of potassium hexafluorophosphate (KPF 6 ). The precipitate was washed with methanol, and the solid was washed with ethanol and CH 3 The CN mixture was recrystallized. Yield: 86%. FABMS: m / e 685; C 42 h 30 N 6 Calculated m / e 684.1 for Zn.
[0057] 1 H NMR (DMSO, δ, ppm): 9.38 (1H, s), 9.12 (4H, d, J=8.0Hz), 8.41 (4H, d, J=7.1Hz), 8.27 (4H, t, J=7.5 Hz), 7.94(4H, d, J=4.2Hz), 7.5(6H, m), 7.48(4H, t, J=6.1Hz). 13 C NMR (DMSO, δ, ppm): 155.1, 149.4, 147.7, 141.2, 135.7, 131.1, 129.8, 129.4, 128.1, 127.6, 123.5, 121.1.
Embodiment 2
[0059] Synthesis of model compound 5b - Yield: 80%. FABMS: m / e 885; C 54 h 54 N 6 o 2 Calculated m / e 884.4 for Zn.
[0060] 1 H NMR (CDCl 3 , δ, ppm): 9.33 (4H, s), 9.14 (4H, d, J=8.0Hz), 8.44 (4H, d, J=8.5Hz), 8.27 (4H, t, J=7.6Hz), 7.93 (4H, d, J = 4.7Hz), 7.48 (4H, dd, J = 12.6 Hz, J = 5.6Hz), 7.29 (4H, d, J = 8.7Hz), 4.17 (4H, t, J = 6.6Hz ), 1.81(8H, m), 1.48(4H, m), 0.92(6H, t, J=6.8Hz). 13 C NMR (CDCl 3 , δ, ppm): 161.9, 155.1, 149.8, 148.3, 141.7, 130.3, 128.1, 127.7, 123.9, 120.4, 115.8, 68.4, 31.5, 29.1, 25.7, 22.6, 14.4.
Embodiment 3
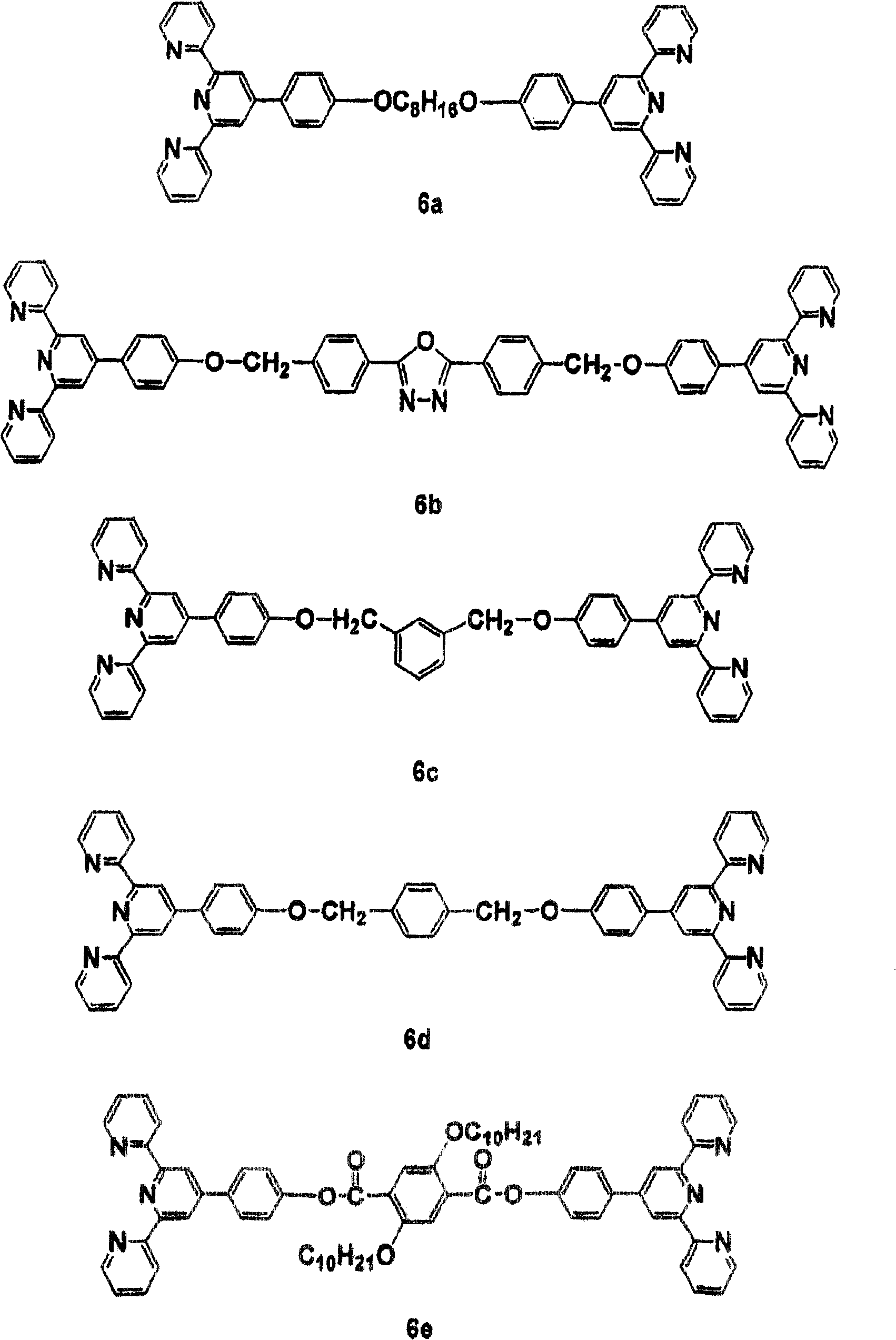
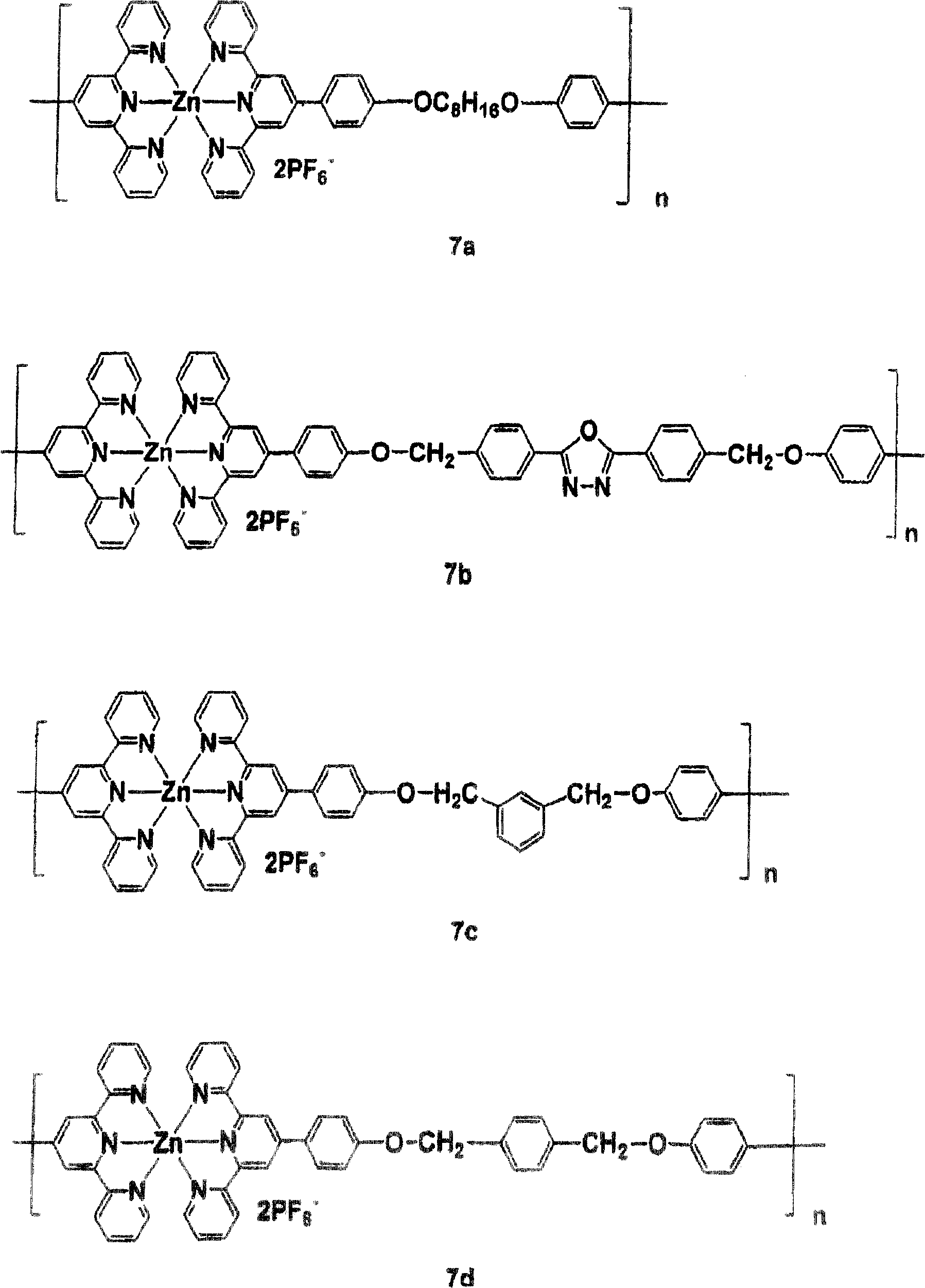
[0062] Synthesis of Monomer 6a - To a suspension of 100 ml DMSO in KOH (2.5 mmol) was added 4'-(4-hydroxyphenyl)-2,2':6',2"-terpyridine (2.05 mmol). Stir at 90° C. for 1 hour, add 1,8-dibromooctane (1.0 mmol) and KI (catalytic amount). The resulting mixture is stirred for 24 hours. The suspension is cooled to room temperature and poured into 500 ml of water. The precipitate is filtered. The resulting The solid was recrystallized from a mixture of ethanol and acetone. Yield: 72%. FABMS: m / e761;C 50 h 44 N 6 o 2 Calculated m / e 760.9.
[0063] 1 H NMR (CDCl 3 , δ, ppm): 8.71 (8H, m), 8.66 (6H, d, J=8.0Hz), 7.86 (8H, m), 7.34 (4H, dt, J=4.8Hz, J=1.0Hz), 7.02 (4H, d, J=8.8Hz), 4.04(4H, t, J=6.5Hz), 1.83(4H, m), 1.50(8H, m). 13 C NMR (CDCl 3 , δ, ppm): 156.4, 155.8, 149.8, 149.1, 136.8, 130.5, 128.5, 123.7, 121.3, 118.2, 118.1, 114.9, 68.1, 29.3, 29.2, 26.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com