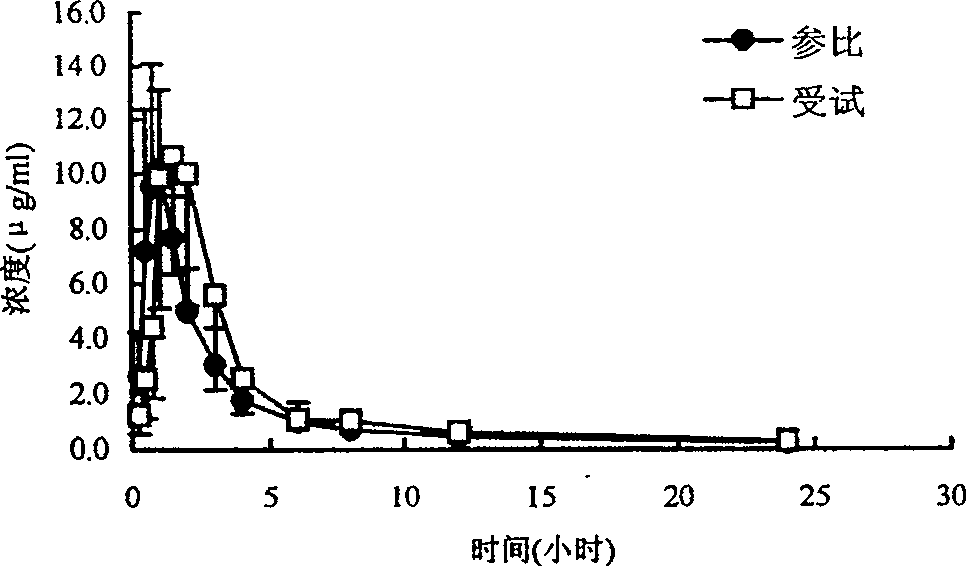
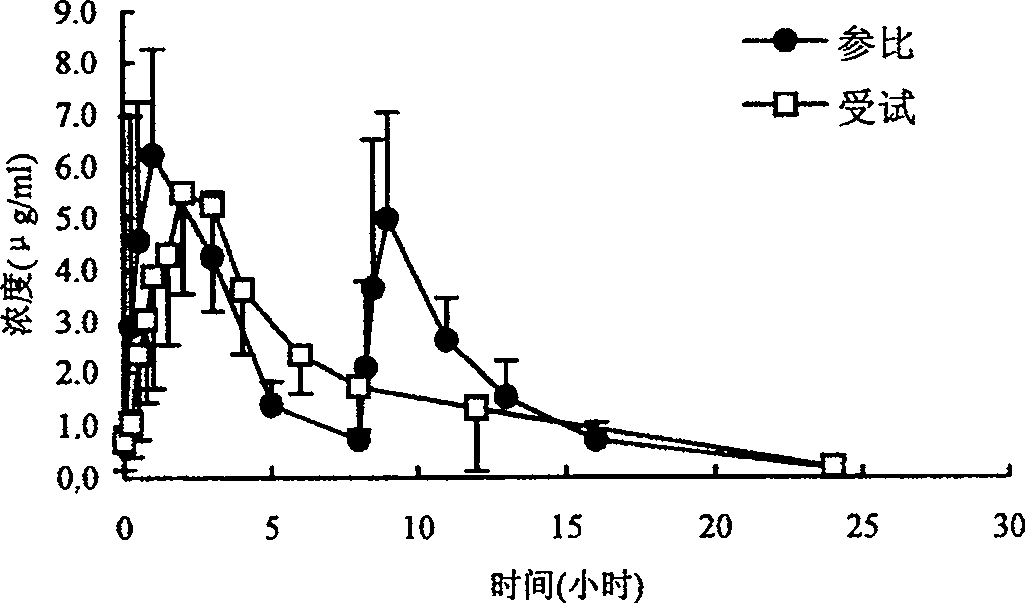
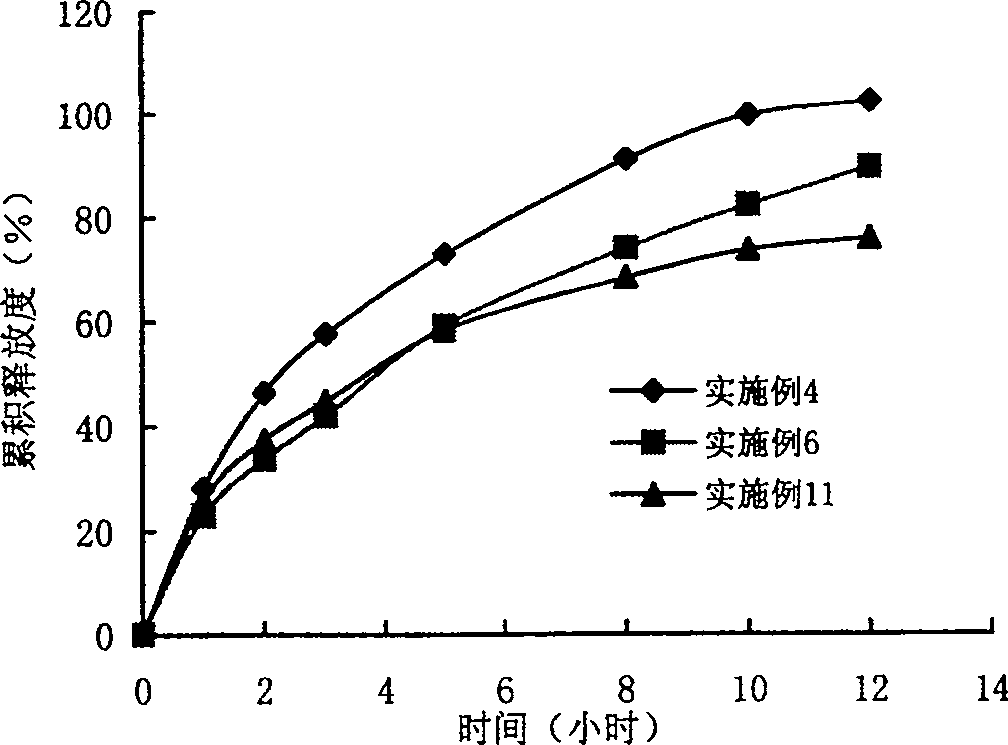
Ranolazine hydrochloride slow-release preparation and its preparing method
A technology for ranolazine hydrochloride and sustained-release preparations, which is applied in the field of oral sustained-release preparations containing ranolazine hydrochloride and its preparation, and can solve problems such as incomplete release of ranolazine base, inability to sustain cardiac protection, incomplete absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Ranolazine Hydrochloride Sustained Release Tablets Formula:
[0107] Each 1000 tablets of Ranolazine Hydrochloride Sustained Release Tablets contains
[0108]
[0109] (1) Pass the formulated amount of hydroxypropyl methylcellulose and ethyl cellulose through a 40-mesh sieve 5 times and mix thoroughly.
[0110] (2) Mix ranolazine hydrochloride with the materials in (1) in equal increments, pass through a 40-mesh sieve 5 times and fully mix.
[0111] (3) Add an appropriate amount of 85% ethanol aqueous solution to the uniformly mixed material, mix uniformly, make a soft material, pass through a 20-mesh sieve to granulate, and dry at 40-50° C. for 2 hours.
[0112] (4) Add the prescribed amount of magnesium stearate, pass the dry granules through a 20-mesh sieve for granulation, fully mix, measure the content of the granules, calculate the tablet weight, and compress into tablets.
[0113] (5) A coating layer can be coated on the outer layer of the sustained-release ...
Embodiment 2
[0115] As described in Example 1, the difference is that hydroxypropyl methylcellulose HPMC K100M CR is replaced by hydroxypropyl methylcellulose HPMC K4M CR, The amount of RS PO was increased to 60 g, and the ethyl cellulose EC10CP STD.PREM.FP was replaced with ethyl cellulose EC100CPSTD.PREM.FP and the amount was reduced to 25.8 g.
Embodiment 3
[0117] As described in Example 1, the difference is that the amount of hydroxypropyl methylcellulose (HPMC K100M CR) is increased to 90g, with RL PO instead For RS PO, the amount of ethyl cellulose was reduced to 27g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com