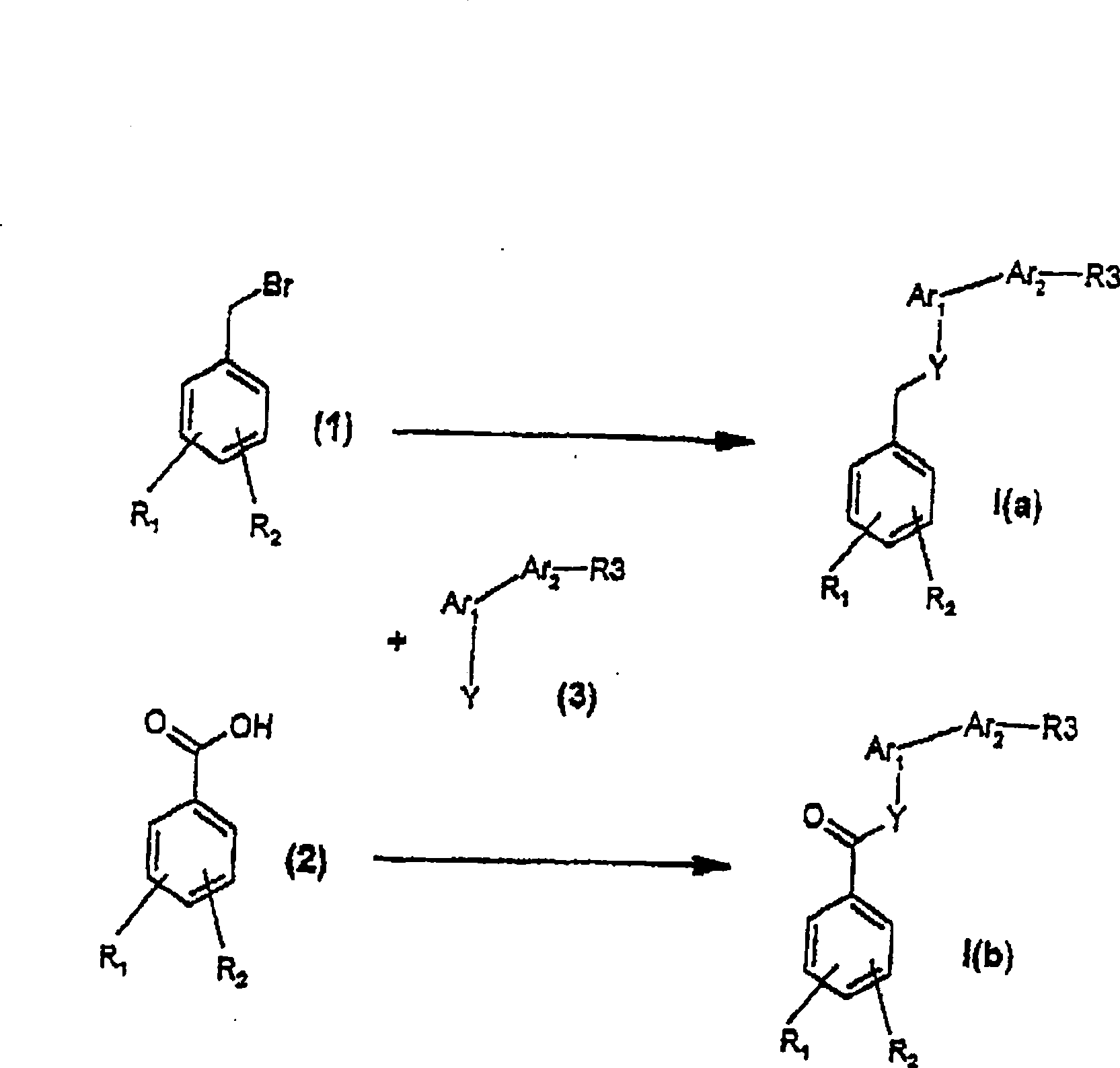
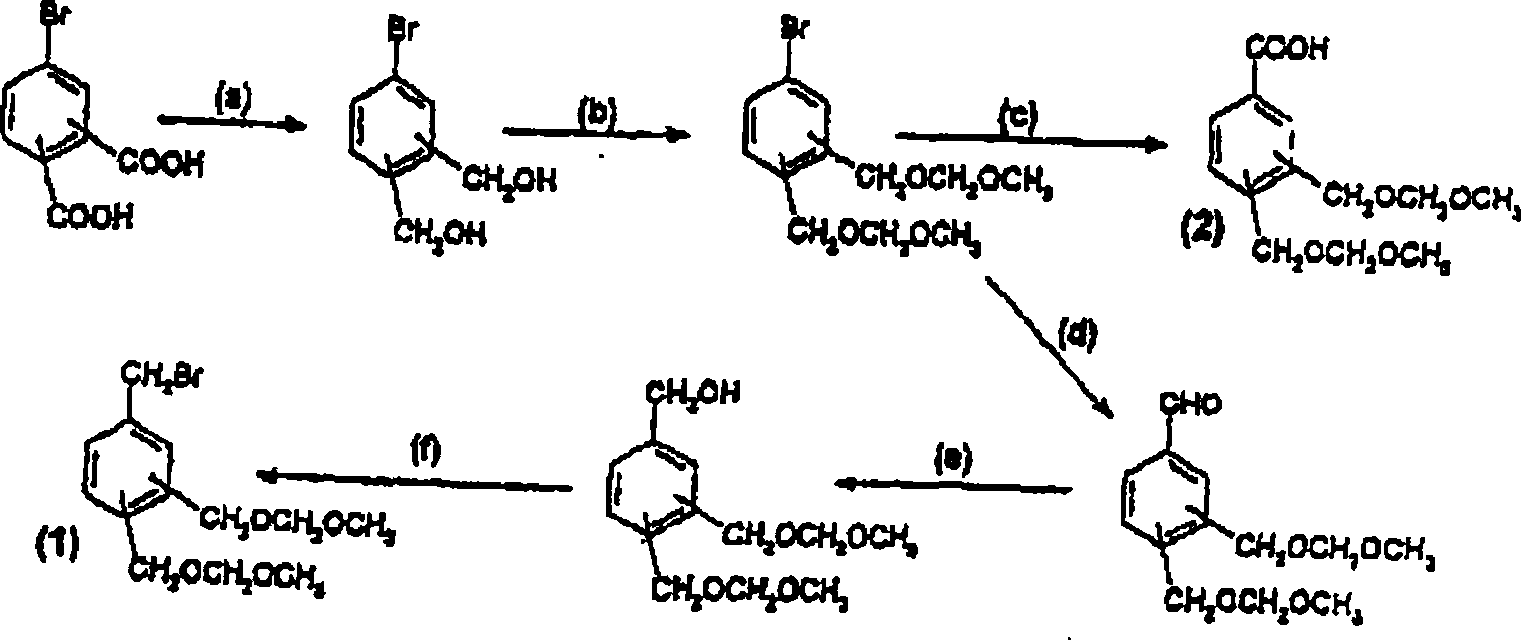
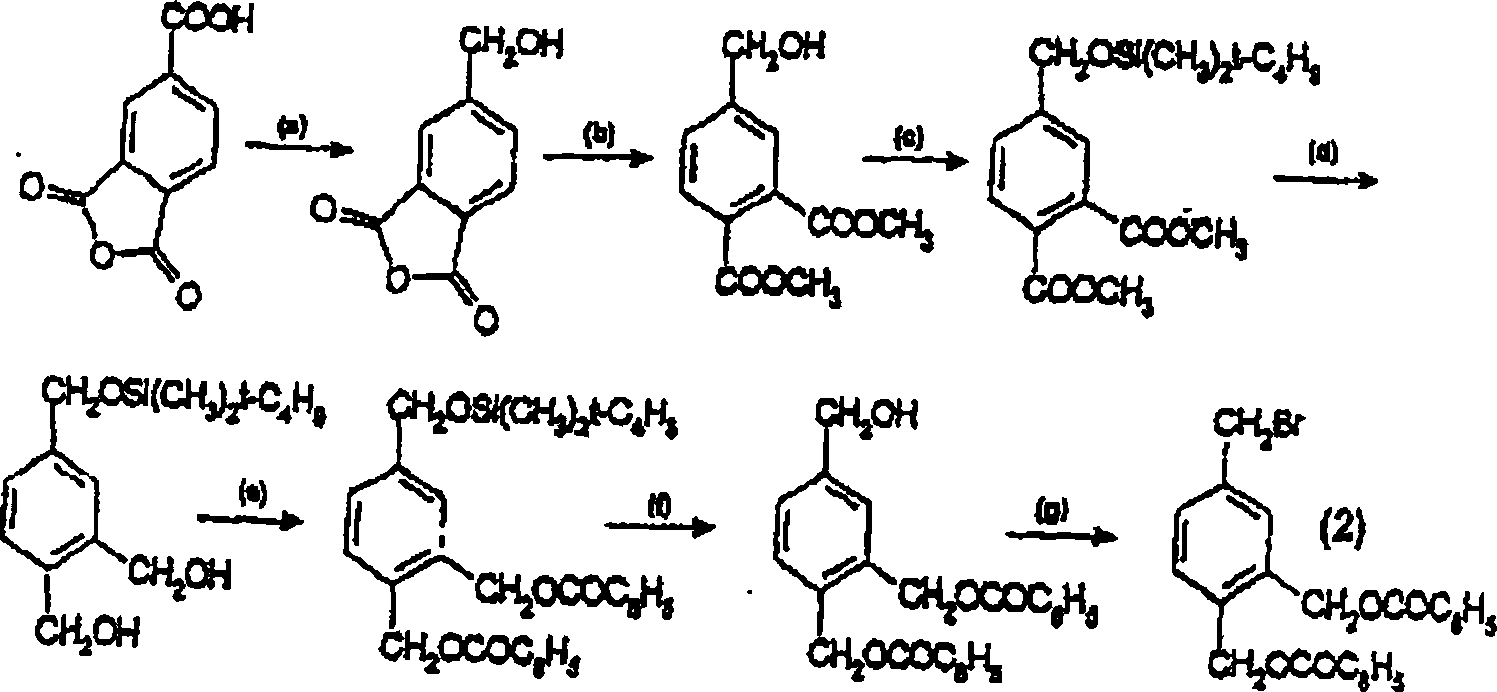
Vitamin D analogues
A compound and composition technology, applied in the field of new practical industrial products, can solve the problems of no effect on calcium metabolism and only retention of differentiation characteristics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] {5-[4,(1-Ethyl-1-hydroxypropyl)biphenyl-3-yloxymethyl]-2-hydroxymethylphenyl}methanol
[0190] a) Dimethyl 4-hydroxymethylphthalate
[0191] 1,2,4-Benzenetricarboxylic anhydride (50 g, 260 mmol) was dissolved in 800 mL of anhydrous dioxane. BH3·THF (260 mmol, 1 eq.) was added dropwise using a dropping funnel at room temperature over about 1 h 30 min. Stirring was continued for 12 hours, and then the reaction solution was poured into a mixture of 600 mL of saturated ammonium chloride solution and 2 L of dichloromethane. After standing to separate the phases, the organic phase was dried and the solvent was evaporated under reduced pressure. To the obtained residue was added 5 mL of sulfuric acid, then dissolved in 1 L of methanol and heated to reflux. After refluxing for 18 hours, the reaction solution was cooled to room temperature and poured directly into a water / ether mixture (1L / 2L). After standing to separate the phases, the aqueous phase was back-extracted wit...
Embodiment 2
[0212] {2-Hydroxymethyl-5-[4'-(1-hydroxy-1-methylethyl)-biphenyl-3-yloxymethyl]phenyl}methanol
[0213] In a manner similar to Example 1(j), 3'-[3,4-bis(benzoyloxymethyl)benzyloxy Ethyl]-biphenyl-4-carboxylate (700mg, 1.16mmol) was purified by silica column chromatography to obtain a white solid (melting point 88-90°C) (m=405mg, Y=93%).
[0214] 1 HNMR (CDCl 3 ): 1.60(6H,s), 2.4(1H,bs), 3.95(2H,bs), 4.70(2H,s), 4.71(2H,s), 5.09(2H,s), 6.92(1H,dd, J 1 =2.5Hz,J 2 =7.2Hz), 7.17-7.19(2H, m), 7.30-7.38(3H, m), 7.43(1H, s), 7.54(4H, s). 13 C(DEPT)32.1(2CH 3 ), 64.1 (CH 2 ), 64.3 (CH 2 ), 70.0 (CH 2 ), 72.7 (C IV ), 113.8(CH), 114.1(CH), 120.3(CH), 125.3(2CH), 127.3(2CH), 127.7(CH), 129.1(CH), 130.2(CH), 130.3(CH), 137.5(C IV ), 139.6 (C IV ), 139.9 (C IV ), 140.5 (C IV ), 142.8 (C IV ), 149.0 (C IV ), 159.4 (C IV ).
Embodiment 3
[0216] {5-[4'-(1-Ethyl-1-hydroxypropyl)-2'-methylbiphenyl-3-yloxymethyl]-2-hydroxymethylphenyl} Methanol
[0217] (a) Ethyl 3'-hydroxy-2-methylbiphenyl-4-carboxylate
[0218] In a manner analogous to Example 1(h), 2 g (13.3 mmol) of 3-methoxymethoxy-phenylboronic acid were reacted with 1.9 g (8.9 mmol) of 3-methyl-4-bromobenzoic acid to obtain 1.6 g (74%) of desired product.
[0219] (b) 3'-[3,4-bis(benzoyloxymethyl)benzyloxy]-2-methylbiphenyl-4-carboxylic acid methyl ester
[0220] In a manner similar to Example 1(j), 1.6 g (6.6 mmol) of 3'-hydroxyl-2-methylbiphenyl-4-ethyl formate was mixed with 3.47 g (7.9 mmol) of 3,4-bis( Benzoyloxymethyl)benzyl bromide was reacted to obtain 3.7 g (94%) of 3'-[3,4-bis(1-phenylformyloxymethyl)-benzyloxy]-2-methyl Methyl biphenyl-4-carboxylate.
[0221] (c) {5-[4'-(1-ethyl-1-hydroxypropyl)-2'-methylbiphenyl-3-yloxymethyl]-2-hydroxymethylphenyl}methanol
[0222] In a manner similar to Example 1(j), 3'-[3,4-bis(benzoyloxymethyl)-benz...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap