Method for killing specific tumour cell by recin
A technology of tumor cells and ricin, applied in the field of genetic engineering, can solve the problems of difficult transfection of adenovirus and restriction of gene transfer of adenovirus vectors, and achieve the goal of less toxic and side effects of the body, large room for improvement, and improved expression specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
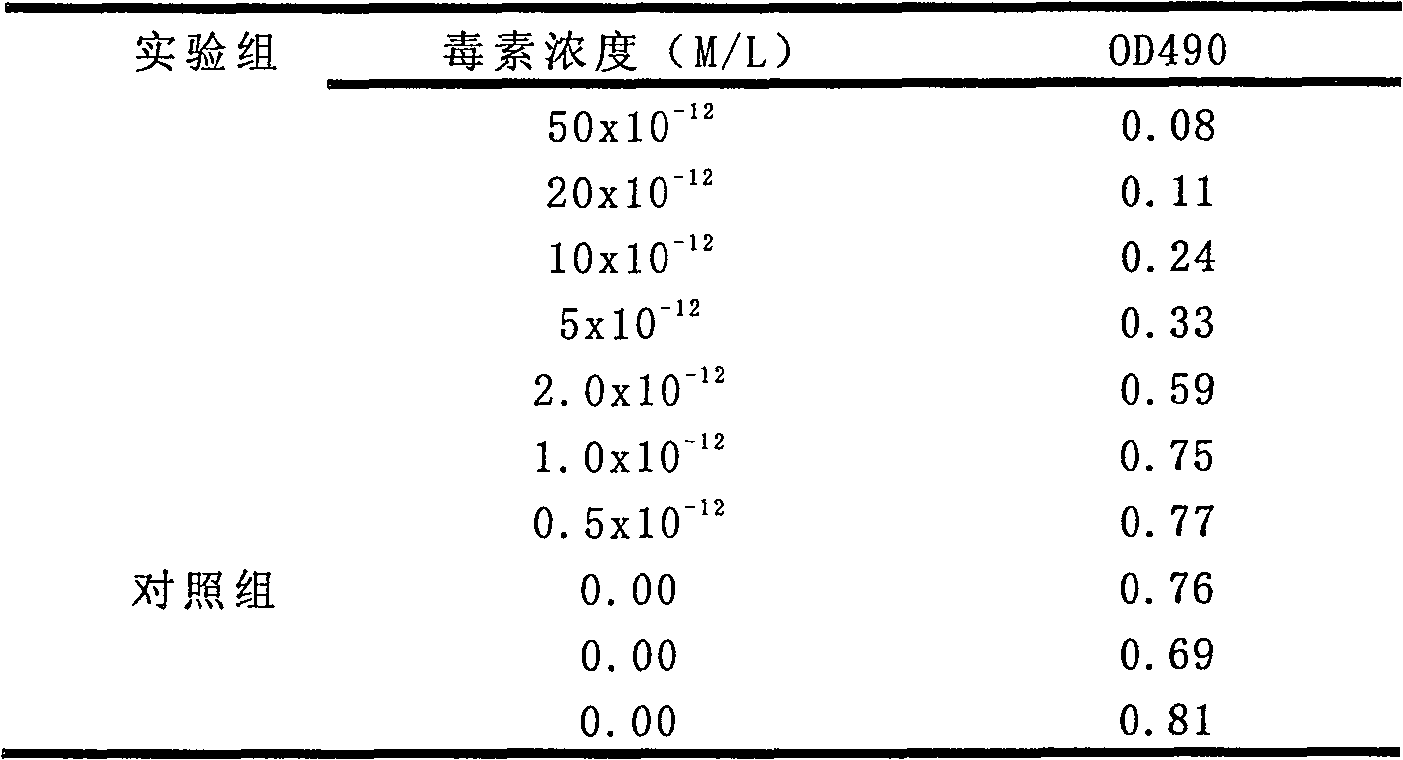
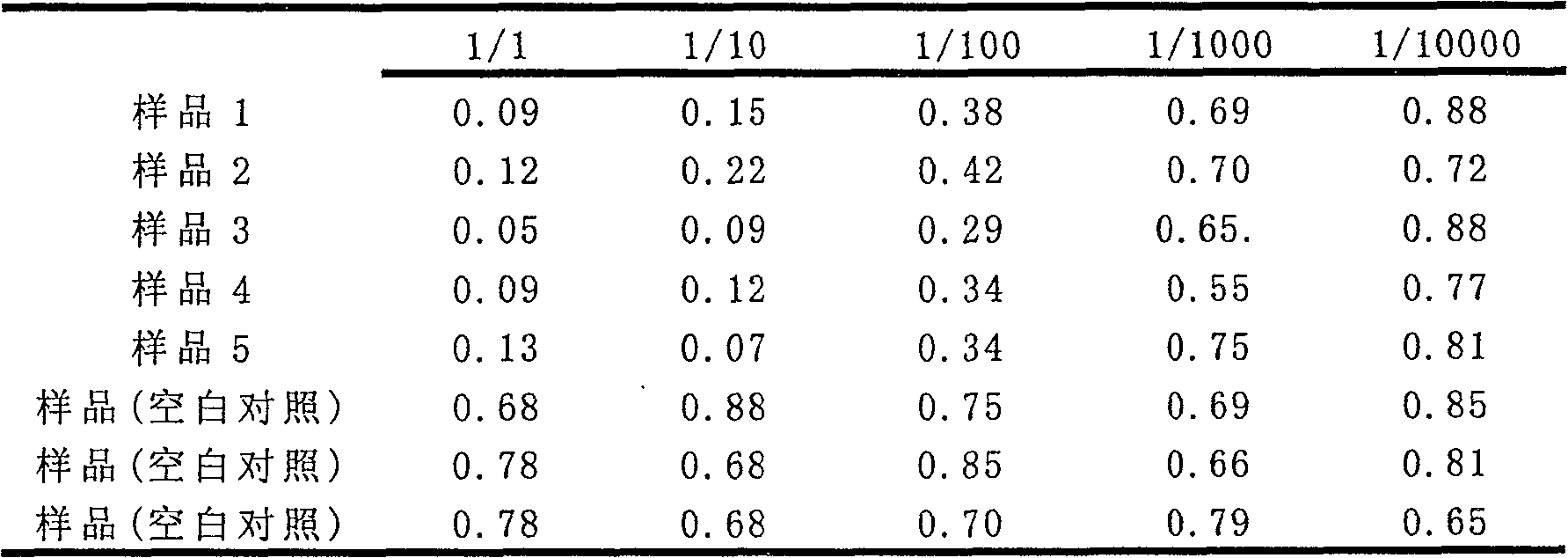
Image
Examples
example 1
[0044] Example one, the cloning of ricin B chain gene
[0045] The top leaves of castor were picked in autumn, and genomic DNA was extracted with the Huashun Bioengineering Company kit according to its instructions. PCR amplification of ricin B chain, PCR primers:
[0046] P15'GTCGACATGGCTGATGTTTGTATGGATCC 3'(Sal I)
[0047] P25'CTCGAGTCAAAATAATGGTAACCATATTTGG 3'(Xho I)
[0048] Using the PCR Kit of TaKaRa Biotechnology Company, according to the instructions provided by the kit, the gene of about 800 bp was obtained by agarose electrophoresis. The gene is installed in the T vector, and the base sequence is the same as that of the ricin B chain gene reported in the literature. The results are shown in SEQ ID NO 1.
example 2
[0049] Example two, ricin, ricin A chain protein purification and activity determination
[0050] Castor seeds were obtained from the Institute of Oil Crops, Chinese Academy of Agricultural Sciences. The seeds are manually dehulled for ricin extraction. The extraction method was carried out in accordance with Biochemistry.1973 for the purification and activity determination of ricin A chain protein. Follow the method described in Tan Lisong's article (Tumor (6) 1986: 363).
example 3
[0051] Example three, construction and identification of E1a-containing adenovirus (AD-E1a-CMV-RTB) containing E1a for expressing ricin B chain using CMV promoter
[0052] The reproductive adenoviral plasmid expressing ricin B chain and E1 deletion was constructed by homologous recombination of pADeasy-1 and pShuttle-CMV viral plasmids purchased from stratagene. The pADeasy-1 adenovirus plasmid is a gene deletion in the E1 and E3 regions, and it is a virus gene that can reproduce in 293 cells. The pShuttle-CMV virus plasmid with multiple cloning sites for inserted genes can be homologously recombined with pADeasy-1 in Escherichia coli BJ5183-AD-1, and the pShuttle-CMV gene can be integrated into the virus genome. The operation of constructing AD-E1a-CMV-RTB is as follows:
[0053] 1. Construction of ricin B chain gene with SV40 replication termination sequence
[0054] Using pShuttle-CMV as a template, the SV40 replication termination sequence was amplified by PCR. The PCR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com