Derivative of folacin alkylation and application
A technology of folic acid alkyl and derivatives, which is applied in organic chemistry, drug combination, liposome delivery, etc., can solve the problems of folic acid leakage and affect the tumor cell targeting effect of folic acid, so as to accelerate cell uptake and improve anti-tumor effect. The effect of tumor therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Synthesis and application of stearic acid derivatives of folic acid
[0021] 1) Synthesis of stearic acid derivatives of folic acid
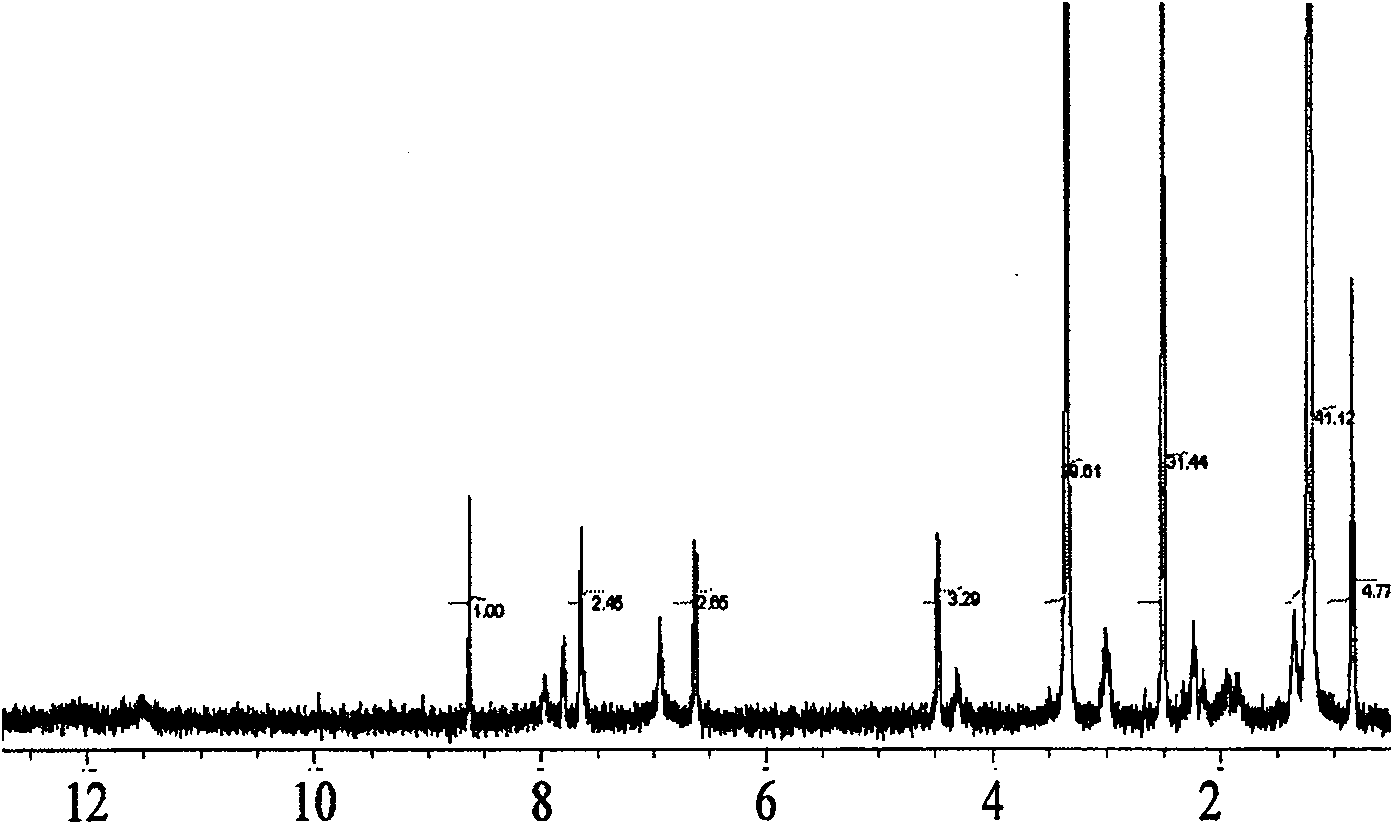
[0022] Accurately weigh stearic acid 20mg and be dissolved in 5ml dimethylformamide (DMF), stir under magnetic force (400r min -1 ), add 20mg of carbodiimide (EDC), after 1 hour of reaction, add 30mg of folic acid and 0.5ml of pyridine, stir and react overnight at room temperature. Add water to precipitate the precipitate, dialyze with distilled water to remove unreacted free folic acid, freeze-dry to obtain the stearic acid derivative of folic acid. For its NMR spectrum see figure 1 .
[0023] 2) Transport of stearic acid derivative lipid nanoparticles containing folic acid in lung cancer A549 cells
[0024] The present invention adopts stearic acid derivative lipid nanoparticle of folic acid containing fluorescein isothiocyanate (FITC) and stearylamine chemical graft to carry out translocation research of lung cancer A...
Embodiment 2
[0039] Embodiment 2: the synthesis and application of stearylamine derivatives of folic acid
[0040] 1) Synthesis of stearylamine derivatives of folic acid
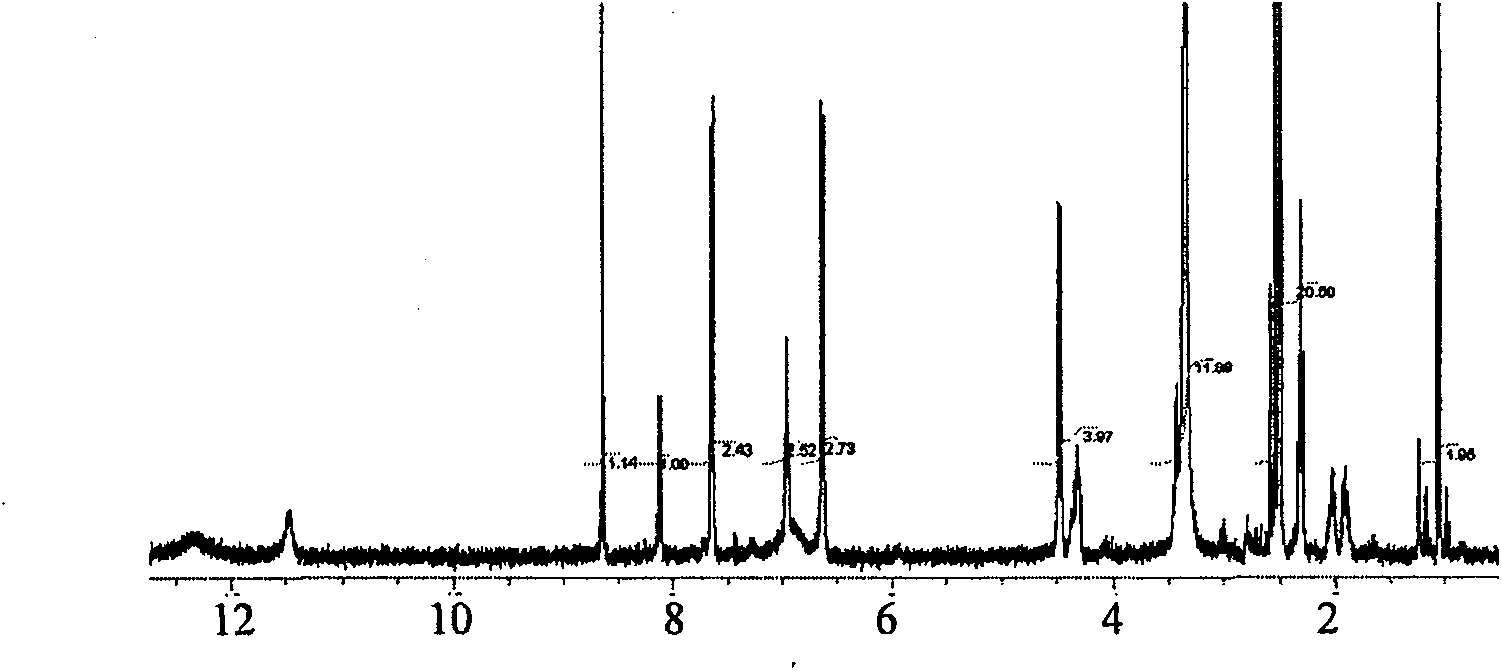
[0041] Accurately weigh stearylamine 20mg and dissolve it in 5ml dimethylformamide (DMF), stir it under magnetic force (400r min -1 ), add 20mg of carbodiimide (EDC), after 1 hour of reaction, add 30mg of folic acid and 0.5ml of pyridine, stir and react overnight at room temperature. Add water to precipitate the precipitate, dialyze with distilled water to remove unreacted free folic acid, and freeze-dry to obtain the stearylamine derivative of folic acid. For the NMR spectra of stearylamine derivatives of folic acid see image 3 .
[0042] 2) Transport of stearylamine derivative lipid nanoparticles containing folic acid in lung cancer A549 cells
[0043] The present invention adopts stearylamine derivative lipid nanoparticles of folic acid containing fluorescein isothiocyanate (FITC) and stearylamine chemical graft ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com